INTRODUCTION
The basic needs of food, shelter, clothing, fuel, ornamentals and flavoring can be provided by plants and they are considered as source of medication for thousands of years1. Over a quarter of world prescribed drugs are derived from plant extracts2. A plant used for the extraction of substances, either for direct medical use or for the synthesis of medicinal compounds which can be used for therapeutic purpose or as a precursor for the synthesis of useful drugs is termed a medicinal plant3. Numerous explorative studies carried out over several decades on certain herbs found them to have definite action on nervous, circulatory, respiratory, digestive and urinary systems; as well as the sexual organs, the skin, vision, hearing and taste4. Other than the beneficial therapeutic effects, these plants may have deleterious side effects as well, ranging from simple electrolyte derangements, architectural distortion of major organs like the liver and kidney, to carcinogenesis in the extreme cases and its consequent cancer development in the future5.
Ziziphus spina-christi commonly known as Christ’s Thorn Jujube is a good example of plants with medicinal effects1,6-8. Important nutrients and phytochemicals in all parts of Z. spina-christi were reported from analysis done by researchers in the past8,9. Betulinic and ceanothic acids, various flavonoids, saponins, erols, tannins, triterpenes, glycoside and flavonoids, lipids, protein, free sugar and mucilage were all extracted from the leaves of the plant9-12.
Among the popular use of Z. spina-christi in traditional medicine is in the treatment of pain and inflammatory related ailments such as arthritis, obesity, diabetes, skin infections, and loss of appetite, fever, pharyngitis, bronchitis, anemia, diarrhea, and insomnia13. The promotion of fresh-wound healing, treatment of dysentery, dandruff, asthma and for the cure of eye diseases are other areas of this plant usage traditionally6,14.
The plant Z. spina-christi has several antioxidants that help in protecting the human body against oxidative damage from reactive free radicals seen in cancers, ischemic heart disease, Alzheimer’s, Parkinson’s disease, atherosclerosis, and aging13. Natural products as a whole and/or their constituents possess efficient ant-oxidative characteristics linking them to anti-cancer, anti-inflammatory, anti-aging and hypolipidemic activities13,15. Fruits from Z. spina-christi inhibit lipid peroxidation in CCl4 exposed animals13. Remarkably, the methanolic extract of the fruits at a dose of 200 mg/kg increases the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSHPx) and GSH in hepatic tissue, all of which are endogenous antioxidant enzymes that protect our system against oxidative stress and its consequent negative effects16. This study aimed to evaluate the acute toxicity potential of Z. spina-christi fruit extracts on Wistar rats.
METHODS
An experimental animal study was carried out to evaluate the acute toxicity potential of Z. spina-christi fruit extracts. Z. spina-christi fruit was purchased dry from Muda Lawan market Bauchi local government area of Bauchi State from the local vendors. The plant was taken to the herbarium in the Department of Biological Science Bauchi State University Gadau, and was authenticated by a taxonomist from the Department of Biological Sciences, Bauchi State University Gadau. The Z. spina-christi fruits were washed to remove dust and then drained. It was then grinded into powder using mortar and pestle and then subjected to extraction using the maceration method.
In brief about 100 g of Z. spina-christi fruit powder were weighed with an analytical weighing balance and transferred to a 1000 mL beaker to which 1000 mL of 80% ethanol were added. Another 100 g of the sample were dissolved in 1000 mL of distilled water. The two beakers containing the mixtures were allowed to stand for 72 h and then filtered using filter paper, and the filtrate was concentrated at 50°C on a water bath. The percentage yield was calculated as follows:
The phytochemical analysis was done for qualitative determination. The analyses included: alkaloids, flavonoids, tannins, terpenoids, saponins, chalcones, phlobatannins, anthraquinones, steroids, phytosteroids, reducing sugars and cardiac glycosides, according the method outlined by the Association of Analytical Chemist17,18.
A total of 15 animals were used for acute toxicity study using OECD 420 guidelines for testing of chemicals, using a fixed-dose procedure with 2000 mg/kg of the extract19. The animals were obtained from animal house of the Department of Pharmacology, Faculty of Basic Medical Science, Bauchi State University Gadau. They were fed with a standard pellet diet and kept under standard environmental conditions of laboratory temperature and water ad libitum. All the animals were housed in polypropylene cages. The animals were kept under alternate cycle of darkness and light of 12 h. They were acclimatized to the laboratory conditions for 2 weeks before starting the experiment. The animals were fasted overnight before the onset of each activity. The animal experimental protocols were approved by Faculty of Basic Medical Sciences’ Research and Ethics Committee (FBMSREC No. BASUG/FBMS/REC/VOL. 2/002) Bauchi State University Gadau. The rats were young adult female albino Wistar rats, nulliparous and non-pregnant. The animals were grouped into 3 cages containing 5 animals each. They were administered with a single oral dose of aqueous and ethanol extracts of Z. spina-christi of 2000 mg/kg and observed for 14 days. The control group received distilled water at 1 mL/kg body weight. The weight of the animals was recorded at days 0, 7 and 14.
At the end of the experiment, the animals were humanely sacrificed. Blood, liver and kidney samples were collected for analysis. The blood was allowed to clot and serum separated and the following investigations were carried out in the serum sample: liver enzymes (AST and ALT) determination of electrolytes (Na, K, Cl–, HCO3–) urea and creatinine and liver proteins (Bil, Con. Bil, Uncon. Bil, total protein, albumin and globulin).
Determination of liver enzymes (AST and ALT)
The liver function test was carried out for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) colorimetrically at 546 nm using a method described by Reitman and Frankel20.
Alanine aminotransferase (ALT)
Alanine aminotransferase was determined using the UV/IFCC method (kit obtained from Randox Laboratories, Ardmore, Co. Antrim, UK). A 500 μL of R1 reagent was pipetted to test tubes labelled blank and test. To which 100 μL of distilled water were added to the test tube labelled blank and 100 μL of sample to the test tube labelled test. Mixed and allowed to stand for exactly 30 min at 37℃. About 500 μL of R2 reagent were added to the test tubes labelled blank and test, mixed and allowed to stand for exactly 20 min at 20–25℃. Then 5000 μL of 0.4N sodium hydroxide were added and mixed. Absorbance was read against reagent blank at 546 nm wavelength after 5 min (calculation: conc. of ALT=test 0.25 × 4).
Aspartate aminotransferase (AST)
Aspartate aminotransferase was determined using the UV/IFCC method using the same kit as before. A 500 μL of R1 reagent were pipetted into test tubes labelled as blank and test. In the test tube blank, 100 μL of distilled water were added and 100 μL of sample to the test tube labelled as test, mixed and allowed to stand for exactly 30 min at 37℃. R2 reagent (500 μL) was added to the test tubes, mixed and allowed to stand for exactly 20 min at 20–25℃. About 5000 μL of 0.4N sodium hydroxide were added and mixed. Absorbance was read against reagent blank at 546 nm wavelength after 5 min (calculation: conc. of AST=test 0.022 × 7).
Determination of plasma urea
The method of Chaney and Marbach21 was used to determined urea concentration (kit obtained from Randox Laboratories, Ardmore, Co. Antrim, UK). Three test tubes were labelled as blank, standard and test, 10 μL of sample were added into the test tube labelled test, 10 μL of standard into the test tube labelled standard and 10 μL of distilled water into the test tube labelled blank. Another 100 μL of reagent I were added to all the three test tubes, mixed and incubated at 37℃ for 10 min. And 2500 μL of reagent II were added to all the three test tubes followed by another 2500 μL of reagent III to all the test tubes, mixed and incubated at 37℃ for 15 min. Absorbance was read at 546 nm against the blank (the color was stable for hours) (urea conc. = absorptance of sample/absorptance of standard × conc. of standard).
Determination of plasma creatinine
The method of Taussky22 was used to determined creatinine concentration. The kit was obtained from Randox Laboratories, Ardmore, Co. Antrim, UK. Test tubes were labelled as test, standard and blank, 100 μL of standard were added to the test tube labelled standard, 100 μL of distilled water to the test tube labelled blank and 100 μL of sample were added to the test tube labelled test. Another 1000 μL were added to all the three test tubes and then mixed. Absorbance was read after 30 s against distilled water (A1). After 2 min absorbance was read against blank at 492 nm [calculation: creatinine conc. = absorptance of sample (A2-A1)/absorptance of standard (A2-A1) × conc. of standard].
Determination of liver proteins.
Liver proteins such as bilirubin and conjugated bilirubin were determined using Randox reagents whereas total protein using biuret reagent and albumin with bromocresol reagents
Determination of total bilirubin
A volume of 200 μL of reagent 1 was pipetted into test tubes labelled blank and test, 50 μL of reagent 2 were also added into the test tube labelled test, and 1000 μL of reagent 3 into the test tube labelled blank. Then, 200 μL of the sample were added into the test tubes labelled blank and test, mixed and allowed to stand for 10 min at 20–25℃. Finally, 1000 μL of reagent 4 into the test tubes labelled blank and test, mixed and allowed to stand for 5–30 min at 20–25℃. Absorbance was read against the sample blank at 578 nm (calculation: total bilirubin conc. (μmol/L) = 185 × absorbance of sample).
Determination of conjugated bilirubin
A volume 200 μL of reagent 1 was added to the test tube labelled blank and test, 50 μL of reagent 2 to the test tube labelled test, 200 μL of 0.9% NaCl to both blank and test. Then, 200 μL of sample were added to the test tubes labelled blank and test, mixed and allowed to stand for exactly 10 min at 20–25℃. Absorbance was read against the sample blank at 546 nm (calculation: direct bilirubin conc. (μmol/L) = 246 × absorbance of sample).
Determination of unconjugated bilirubin concentration
Plasma indirect bilirubin concentration was estimated using the formula: Indirect bilirubin conc (μmol/L) = total bilirubin - direct bilirubin.
Determination of total protein
Total plasma protein (TP) values were measured colorimetrically at 546 nm using the biuret method23. A 1000 μL of biuret reagent were added into three test tubes labelled blank, standard and test. Then, 10 μL of standard were added into the test tube labelled standard, 10 μL of serum to the test tube labelled test, mixed well and incubated for 5 min at room temperature. Absorbance of standard and sample was read against reagent blank within 60 min at 546 nm. (calculation: conc. of standard = 6 g/dL. Total protein conc. (g/dL) = absorptance of test/absorptance of standard × 6).
Determination of albumin
Test tubes were labelled as blank, standard and test, 1000 μL of reagent were added to all the three test tubes and 5 μL of standard to the test tube labelled standard, 5 μL of sample to the test tube labelled test, mixed and incubated for 10 min at room temperature (15–25℃). Absorbance of the sample and standard were read against the blank at 630 nm. The color was stable for 60 min at room temperature (calculation: conc. of standard = 5 g/dL. Conc. of albumin = absorptance of sample/absorptance of standard × conc. of standard).
Determination of globulin
Plasma globulin concentration was determined using the formula: Globulin = Total serum protein - albumin.
Histopathology of the liver and kidney
All the rats were sacrificed by exsanguinations after light ether inhalation anesthesia at the end of the experiment. The animals were subjected to post-mortem examination with collection of organs such as liver and kidneys. The tissues were fixed in 10% formalin for at least 48 h, in 4–5 μm paraffin sections and processed and stained with hematoxylin and eosin (H&E) for microscopic examination using established protocols24.
Statistical analysis
Statistical analysis was carried out using mean ± standard error of mean (SEM) and analyzed with standard statistical software package for social science (SPSS) version 17.0 software using one-way analysis of variance (one-way ANOVA) and Dunnett’s post hoc test for differences between multiple and two means, respectively. Differences were considered statistically significant at p<0.05. Bar charts were drawn using Exel 2013 software.
RESULTS
The percentage yield of aqueous extract was 35.59% and that of ethanol extract 46.68%. Alkaloids, steroids, saponins, flavonoids, terpenoids, saponins glycoside, phytosteroids, cardiac glycosides, tannins, volatile oil and carbohydrate in different proportion were the secondary metabolites obtained from the phytochemical analysis of the aqueous and ethanol extract of fruits of Z. spina-christi. Details of the phytochemicals obtained are shown in Table 1.
Table 1
Qualitative phytochemical contents of Zizipus spina-christi fruit aqueous and ethanol Extracts
An acute toxicity using OECD guidelines was done with a single oral dose exposure of both the aqueous and ethanol extracts of Z. spina-christi. Animal treated with 2000 mg/kg of the two extracts showed no sign of toxicity within the 14 days of study. No animal died during the observation and no gross change in morphology was seen on all the major organs observed after the acute toxicity study. Figure 1 shows the weight of the animals treated with 2000 mg/kg of aqueous and ethanol extracts of Z. spina-christi for 14 days. The weight was recorded on days 0, 3, 7 and 14.
Figure 1
Effect of Ziziphus spina-christi fruit aqueous and ethanol extracts on the body weight in experimental rats
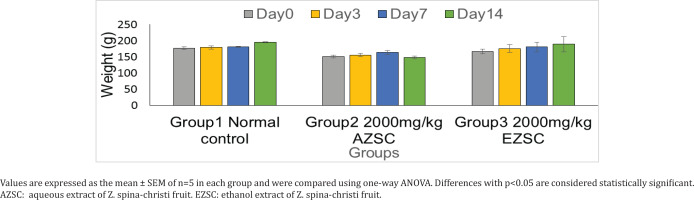
Table 2 shows effect of aqueous and ethanol extract of Z. spina-christi fruit on experimented rats on liver enzymes treated with 2000 mg/kg for 14 days. There is a significant difference between the normal control group and the treated group in alanine aminotransferase but no significant difference in aspartate aminotransferase. The AST/ALT ratio in the normal control group was 5:1 whereas in the treated group it was 2:1.
Table 2
Effect of Zizipus spina-christi fruit aqueous and ethanol extracts on liver enzymes in experimental rats
| Groups | ALT | AST | AST/ALT |
|---|---|---|---|
| Normal control | 4.13 ± 0.23* | 22.49 ± 3.83 | 5:1 |
| AZSC 2000 mg/kg | 13.60 ± 0.40* | 22.17 ± 1.43 | 2:1 |
| EZSC 2000 mg/kg | 12.11 ± 0.70* | 21.43 ± 2.12 | 2:1 |
Concentration of total bilirubin, conjugated bilirubin, unconjugated bilirubin, total protein, albumin and globulin of experimental rats treated with 2000 mg/kg body weight of Z. spina-christi fruit aqueous and ethanol extracts are presented in Table 3. In general, there is an increase in all the parameters tested in the two treated groups compared to the normal saline control group as shown in Table 4.
Table 3
Effect of Zizipus spina-christi fruit aqueous and ethanol extracts on liver proteins in experimental rats
[i] Values are expressed as the Mean ± SEM of n=5 in each group and were analyzed using one-way ANOVA. Differences with p<0.05 are considered statistically significant. Values with the same superscript (* or a) are significantly different from the normal control group. AZSC: aqueous extract of Z. spina-christi fruit. EZSC: ethanol extract of Z. spina-christi fruit.
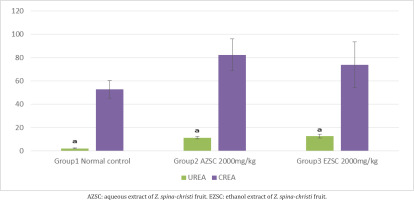
Urea and creatinine concentrations of experimental rats treated with 2000 mg/kg aqueous and ethanol extracts of Z. spina-christi fruit after 14 days of observation were determined. There is an increase in both the biomarkers, with a statistically significant difference to the normal control group. The details are shown in Figure 2.
DISCUSSION
Whole plants and most of their parts have been in use for many years for their nutritional and medicinal values making them a part of alternative medicine, food pharmaceuticals, and natural therapies1,2. Z. spina-christi fruit is an example of such plant with high nutritional and medicinal values1,6-8. Presence of various secondary metabolites such as alkaloids, flavonoids, glycosides and tannins is believed to be reasons for the therapeutic properties of Z. spina-christi fruits13. Alkaloids, steroids, saponins, flavonoids, terpenoids, saponins glycoside, phytosteroids, cardiac glycosides, tannins, volatile oil and carbohydrate in different concentration were the metabolites obtained from the phytochemical analysis of the aqueous and ethanol extracts of fruits of Z. spina-christ. The values of alkaloids, tannins, steroids, cardiac glycosides and volatile oil were shown to be higher in the ethanol extract than the aqueous extract. While saponins and terpenoids were more in aqueous than the ethanol extract. The concentration of carbohydrate and flavonoids were shown to be equal in the two extract solvents. This variation in the concentration of secondary metabolite in aqueous and ethanol extracts may be attributed to the polarity of the two solvents. This result is consistent with the finding of Trung et al.25 on Severinia buxifolia that showed differences due to differences in the polarity of the extract solvents. Othe studies1,6-9,14,26-28 examined different parts of the plant including leaves, stem bark, root and seed oil and showed the presence of most of these compounds in different solvents. However, no volatile oil was seen in their extract, which may be attributed to the volatile nature of the compound.
An acute toxicity with a single oral dose of aqueous and ethanol extracts of Z. spina-christi fruit at 2000 mg/kg showed no sign of toxicity such as convulsion, vomiting, bleeding, breathing difficulties within the period of 14 days study. No animal died during the observation and no gross change in morphology was seen on all the major organs. This finding is in agreement with that of Abubakar et al.29 who measured the LD50 of the hydro-methanol extract of the fruit at a concentration of 5000 mg/kg29. They recorded zero toxicity, and no pathological changes on any major organ were seen with the naked eye.
The weight was recorded on days 0, 3, 7 and 14, and showed no significant difference. The rats continued to gain weight throughout the study period, the gain was more sustained in the control group than the two treatment groups. The food intake and water were also observed to be unaffected by the treatment. A weight reduction of 10% or more is usually considered very significant and usually depicts severe disease for which the toxicity of drug is one of them30.
Alanine aminotransferase and aspartate aminotransferase are important biomarkers of the liver. Elevated levels may indicate an abnormality such as hepatocellular injury due to either physical or chemical injuries31. The effect of aqueous and ethanol extracts of Z. spina-christi fruit on experimental rats after a single dose of 2000 mg/kg for 14 days, demonstrated significant differences between the normal control group and the treated group in alanine aminotransferase only, but not the aspartate aminotransferase. The AST/ALT ratio in the normal control group was 1:5 and in the treated groups was 1:2. The reverse of this enzyme ratio results from an increase in the level of ALT after the 2000 mg/kg dose of the extracts. ALT is an enzyme that is more specific to liver insult seen in drug toxicity, alcohol and many inflammatory conditions of the liver. This result agrees with the finding of Marghoob et al.31 who analyzed serum levels of alanine and aspartate aminotransferases, alkaline phosphatase and gamma glutamyl transferase, and concluded that comparative elevation indicates the degree of hepatic damage in viral hepatitis, alcoholic liver diseases, and cirrhosis. Sunmonu and Afolayan32 similarly mentioned that elevation in ALT and AST suggested damage to the liver cells.
Concentration of total bilirubin, conjugated bilirubin, unconjugated bilirubin, total protein, albumin and globulin of experimental rats treated with 2000 mg/kg body weight of Z. spina-christi fruit aqueous and ethanol extracts shows an increase in all the parameters. Bilirubin is an important metabolic breakdown product of blood with biological and diagnostic values. It is removed from the body by the liver; hence, it is a good indicator of the health status of the liver32. Albumin and globulin are mixtures of protein molecules that are used to assess the health status of the liver. The two proteins are produced by the liver; albumin is a major carrier of drugs and many minerals that circulate in the bloodstream; globulins are larger proteins responsible for immunological responses32. The elevated serum level of bilirubin in experimental rats, as observed in the present study, may be a result of reduced uptake arising from liver disease.
Kidneys maintain chemical composition of body fluids by acidification of urine, and some excretal substances are released into the urine by active transport in the renal tubules. These substances include H+ and K+ ions, urea, and creatinine, as well as drugs such as penicillin. An elevated level of urea and creatinine are significant markers of kidney dysfunction indicating an inactivity in glomerular filtration rate33. The urea and creatinine concentrations of experimental rats treated with 2000 mg/kg aqueous and ethanol extracts of Z. spina-christi fruit after 14 days of observation show a significant elevation in both the biomarkers. These results suggest that a high dose of the Z. spina-christi fruit might be toxic to kidneys, even though the concentrations of urea and creatinine are within the normal range.
Liver histology was done using H&E stain to show distortion or otherwise of the liver architecture after 14 days study of the rats treated with both ethanol and aqueous extracts compared to normal control group (Supplementary file Figures 3–8). The liver architecture is well preserved in the normal control group. The central vein is clearly delineated surrounded by many sinusoids and few inflammatory cells. The nucleus was clearly seen and the nuclear cytoplasmic ratios are equal in most cell within the view. However, the liver architecture of the two treated groups was distorted. Tissue necrosis is seen as evidence by perinuclear vacuolation; also present are few inflammatory cells in the aqueous extracted treated group. The picture of ethanol treated rats is seen with some distortion as well, there are few inflammatory cells, the nuclei are moderately pyknotic and the sinusoids are markedly reduced in space. This finding supports the liver enzyme and liver protein analyses of the study, both of which showed derangement after 14 days of study. The hepatocytes, portal triads and the sinusoids are usually the major parts of liver vulnerable to histological changes in liver toxicity33. Anisokaryosis, nuclear vesiculation, binucleation, cytoplasmic inclusions, cytoplasmic swelling, hydropic degeneration, necrosis and reduction in glycogen content were seen in lead toxicity of the liver34. Kupffer cells hyperplasia and occasional fatty changes of the portal triads together with hemosiderosis are also frequently seen.
Kidneys of the experimental rats from the control group show normal morphology as demonstrated by the presence of glomeruli surrounded by clear zones of capsular spaces and the glomerular capsules are intact. However, rats treated with a single dose of 2000 mg/kg aqueous extract of Z. spina-christi fruit showed glomerular atrophy and generalized tissue degeneration as seen by the dissolution of both nuclear and cytoplasmic components. Due to the degeneration, the nuclei are presented as naked nuclei. There is a lot of inflammation as demonstrated by the presence of several inflammatory cells within the tissue. Collagens deposition was seen in significant quantity around the glomerulus and the kidney tubules. Kidneys of experimental rats treated with 2000 mg/kg ethanol extracts also showed generalized tissue degeneration demonstrated by the presence of naked nuclei and the complete loss of nuclei in some cells. Increase in tubular spaces indicating tissue atrophy and mild collagenation shown by the presence of collagen fibers around the tubules are seen. The results of the two kidneys seen from histology, added weight to the increase in urea and creatinine obtained from blood analysis. Thus, both ethanol and aqueous extracts of this plant at the tested doses cause some liver and renal toxicity.
The same plant tested for toxicity in rats, at doses lower than ours given daily for 14 days, demonstrated a significant (p<0.05) reduction in the levels of white blood cells, neutrophils, serum aspartate amino transferase, Cl−, urea and creatinine. The lymphocytes, platelets, direct and total bilirubin, albumin, alanine amino transferase, alkaline phosphatase, aspartate aminotransferase, serum Ca2+, creatinine, and urea of rats were all elevated35. The liver and kidney architecture was not altered after the histological studies. This is likely due the use of doses lower than 2000 mg/kg used in this study.
Limitations
The present study has several limitations that should be acknowledged. Firstly, the study used a qualitative phytochemical analysis approach, which did not provide quantitative data on the bioactive compounds present in the fruit extracts. Additionally, the study only evaluated the effects of the fruit extract on liver and kidney functions in Wistar rats, and did not investigate other potential toxic effects on other organs or systems, or other animal models. Furthermore, the study had a limited sample size, which may impact the generalizability of the findings. Finally, the study used dried fruit purchased from local vendors, which may vary in quality, ripeness, and processing, potentially affecting the results.
CONCLUSIONS
Our study provides valuable insights into the phytochemical composition and toxicological effects of Z. spina-christi fruit extracts. The findings indicated that it can be toxic at higher doses. Specifically, our results demonstrated that high doses of the fruit extract can cause significant alterations in liver enzymes, urea, creatinine, and liver proteins, as well as histological changes in the liver and kidneys. The study contributed to the growing body of knowledge on the potential uses and limitations of Z. spina-christi fruit extracts. Our findings highlight the importance of thorough toxicological evaluation of traditional remedies and provide a foundation for future studies to build upon. The study’s use of dried fruit from local vendors also reflects the typical consumption habits of the local community, making the findings more relevant and applicable to real-world scenarios. Overall, our research emphasizes the need for a balanced approach to traditional medicine, combining the benefits of natural remedies with rigorous scientific evaluation to ensure safe and effective use.