INTRODUCTION
Human exposure to pesticides, including herbicides, has been linked to the development of a number of diseases1-3. Glyphosate (N-(phosphonomethyl) glycine) is the active ingredient in various herbicides, of which the most widely used is Roundup®. The use of Roundup has been linked to the development of non-Hodgkin lymphoma among agricultural workers4,5. In addition, some toxicity tests performed in laboratory animals and cell model systems suggest that glyphosate can be carcinogenic6,7. It is suspected that glyphosate affects the respiration of mitochondria in animal cells, generating oxidative stress which can lead to DNA damage6,7. This weight of evidence was used by the International Agency for Research on Cancer (IARC) to conclude that glyphosate is a probable human carcinogen4. However, it is not clear whether effects are arising from glyphosate or from other compounds present in Roundup herbicides, or from their interactions.
Since glyphosate is declared as the active herbicidal ingredient, all other ingredients entering the composition of Roundup herbicides are considered inert and ignored in safety evaluations, even if a large number of studies have shown their toxic effects8. Many studies have demonstrated the toxic effects of co-formulants included in the formulations of glyphosate herbicides8. However, very little is known about other pesticides, which can be mixed with glyphosate. These include the herbicides 2,4-D (N-(phosphonomethyl) glycine) and dicamba (3,6-dichloro-2-methoxybenzoic acid), the use of which has been increasing markedly in recent years due to the introduction of new generation genetically modified (GM) crops that are tolerant to glyphosate plus dicamba or glyphosate plus 2,4-D, in an effort to counter the widespread emergence of glyphosate-resistant weeds.
Dicamba is a benzoic acid used as a selective post-emergent broad-leaf herbicide. Dicamba-tolerant GM crops (e.g. Xtend soybeans) are commercialized in the US. Like dicamba, 2,4-D mimics the natural auxin indole-3-acetic acid (IAA), interfering with plant phytohormone responses. Chlorophenoxy herbicides such as dicamba and 2,4-D are toxic to mammals through different mechanisms, including uncoupling of oxidative phosphorylation and alterations of acetyl-coenzyme A metabolism causing a large range of acute adverse effects9. However, little is known about the effects of these pesticides during sensitive periods of development, especially in which way the physiological changes caused by in utero exposure can alter metabolism and predispose to the development of diseases during adulthood.
Usually, most of the studies focus on determining detrimental effects caused by exposure to one or two substances. However, in reality, humanity is being exposed daily to a wide variety of chemicals, most of which are potential or indeed toxic substances. What is of concern is that it is extremely difficult to comprehensively know the effects that simultaneous exposure to several chemical substances can induce10. It is proven that the endocrine system is the first to be affected by the majority of these substances causing problems in all systems of the organism through hormonal malfunction. Nevertheless, researchers have introduced the concept of simulating real-life risks, trying to identify health impacts11. Low or long-term exposure of experimental animals to mixtures of pesticides, plasticizers, antimicrobial agents, and other food additives has given interesting results concerning multi-health impacts from this type of exposure12-14.
In this project, we will measure the genotoxicity of glyphosate at regulatory relevant doses namely the European Union (EU) acceptable daily intake (ADI) and no-observed adverse effect level (NOAEL) in Wistar rats exposed, starting prenatally to early adulthood. Rats will also be exposed to a mixture of glyphosate, 2,4-D, and dicamba all at their respective ADI, in order to mimic exposure arising from the introduction of GM crops engineered to be tolerant to a combination of these herbicides. Similar protocols have been published lately, trying to emphasize the importance of studies simulating real-life risks15.
METHODS
Three-month-old Wistar rats bred within the Animal House of the University of Medicine and Pharmacy of Craiova, will be used in this investigation. Animals will be kept under standard conditions, including a twelve-hour light/dark cycle, a controlled room temperature of 19–23oC, and 35–55% humidity. Animals will have ad libitum access to food and filtered tap water, which will contain the test substances, where appropriate.
Glyphosate approved as pesticide under Reg. (EC) No 1107/2009 will be administered at the EU ADI (0.5 mg/kg bw/day), and NOAEL (50 mg/kg bw/day)16. A group of rats will be exposed to a mixture of glyphosate (0.5 mg/kg bw/day), 2,4-D, approved as pesticide under Reg. (EC) No 1107/2009 (0.02 mg/kg bw/day)17 and dicamba, approved as pesticide under Reg. (EC) No 1107/2009 (0.3 mg/kg bw/day)18, which corresponds in each case to the EU ADI.
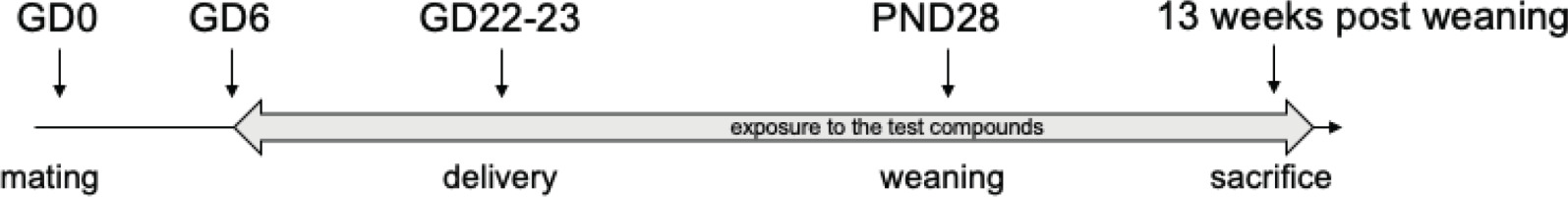
We will follow the dosing strategy recommended by Organization of Economic Cooperation and Development (OECD) guideline TG 414 and start the experiment at gestational day (GD) 6. The experiment will be terminated 13 weeks after weaning (about 17 weeks) in order to comply with 90-day feeding study requirements (Figure 1).
Exposure to glyphosate and the glyphosate/2,4-D/dicamba herbicide mixture will start at gestational day 6 (GD6) and continue throughout the period of gestation and lactation up to post-natal day 28 (PND28), and with the off-spring to 13 weeks post-weaning when animals will be sacrificed for analysis.
The administration of the test compounds will start on gestational day 6 (GD6) and continue throughout gestation and lactation (Figure 1). Twenty pregnant rats will be divided into 4 groups and treated with the corresponding doses of test compounds. After weaning, the pups from each treatment and control group will be randomized to achieve homogeneous body weights across the groups. From the pups, a total of 80 pups (40 males and 40 females, 2 males and 2 females from each litter), will continue with the treatment corresponding to their group for another 13 weeks post-weaning. We will analyze 10 males and 10 female rats per group. The remaining pups from each group will be divided into an additional set of 4 groups of rats that will be kept for 3 months on standard diets in order to evaluate if exposure during pregnancy can have consequences on adult physiology, even if animals were subsequently not exposed during the first few months of life.
After the exposure period, the animals will be sacrificed by exsanguination after anesthesia with a mixture of 9.1 mg/kg bw xylazine (Alfazyne 2%, Alfasan Int., Woerden, Netherlands) and 9.1 mg/kg bw ketamine (Alfamine 10%, Alfasan Int., Woerden, Netherlands). At sacrifice, multiples of tissue (100 mg) samples from the same major organs (Table 1) will be snap frozen. This will allow the realization of different omics analyses, as well as the storage of backup samples. Organ harvest will include liver, kidneys, lymph nodes, bone marrow, brain regions (cortex, cerebelum, hypocampus) and pancreas, as well as the small and large intestines. The content of the caecum and of the small intestine will also be collected in order to conduct microbiome analyses.
Table 1
Summary of the sampling strategy
DISCUSSION
Intestinal barrier integrity
Assessment of intestinal barrier integrity will be performed using real-time quantitative polymerase chain reaction (RT-qPCR). The expression of 5 genes indicative of alterations in the integrity of tight junctions will be measured in the large intestine (caecum) and ileum. Extracted RNA is retrotranscribed to cDNA using the SuperScript VILO cDNA Synthesis Kit (ThermoFisher Scientific, Loughborough, UK). A total of 100 ng cDNA is then amplified using TaqMan assays for Muc2 (Rn01498206_m1), Ocln (Rn00580064_m1), Zo1 (Rn07315717_m1), Cldn3 (Rn00581751_s1), and Cldn4 (Rn01196224_s1). Additionally, the expression of 3 genes, namely Il22 (Rn01760432_m1), Lcn2 (Rn00590612_m1) and Tlr4 (Rn01458370_m1), indicative of the occurrence of inflammation, will also be assessed in both the large intestine and ileum. All RT-qPCR assessments of target mRNA levels will use Gapdh (Rn01775763_g1) as an internal reference standard. Reactions are conducted as technical duplicates, with a TaqMan Fast Advanced Master Mix (ThermoFisher Scientific, UK) on the Applied Biosystems QuantStudio 6 Flex Real-Time quantitative PCR (RT-qPCR) System. The delta–delta Ct method is used to calculate the relative gene expression of the target genes. Intestinal inflammation is further investigated by determining calprotectin levels in caecum content using the Immundiagnostik S100A8/S100A9 ELISA kit (Immundiagnostik, Bensheim, Germany). Briefly, a 100 μL aliquot of caecum content and standards provided in the kit are mixed with 100 μL of detection antibody in the coated ELISA kit 96 well microtitre plate and incubated for 1 hour at 37°C. After washing, 100 μL substrate is added at 37°C. The absorbance is then read at 450 nm, after adding stop solution, with a microplate reader (Promega, UK). Calprotectin levels are expressed as ng/mL caecum content.
Gut microbiome evaluation
The bacterial and fungal composition of the gut microbiome will be analyzed by analyzing amplicons from the 16S rRNA and ITS2 genes, respectively, as previously described19.
Hormonal determination
Serum levels of total T3 (triiodothyronine), total T4 (thyroxine), TSH (thyroid stimulating hormone) and testosterone will be determined in serum samples by ELISA according to the manufacturer instructions using the total T3 kit (catalog no. EIA-4569, DRG Instruments GmbH, Marburg, Germany), total T4 kit (catalog no. EIA-4568, DRG Instruments GmbH, Marburg, Germany), TSH kit (catalog no. EIA-1782, DRG Instruments GmbH, Marburg, Germany), and testosterone kit (catalog no. EIS-1559, DRG Instruments GmbH, Marburg, Germany).
Motor and neurobehavioral evaluation
Assessment of motor performance will employ the rotarod test, which is appropriate for assessing rat coordination and sensorimotor performance in accelerating rotating rod mode of function. The rotarod device is a horizontally rotating rod about 30 cm long and 6 cm in diameter. It is connected to a motor with two speed settings: 1) constant 5 rpm, and 2) adjustable 4–40 rpm in 300 seconds. The pole hangs 27 cm above the landing table. The animals should be brought into the testing room at least one hour before the test for acclimatization. All rotarod procedures will be performed at the same time of day for all animals in each group. The rotarod experiment consists of an initial training phase followed by a test phase. The training phase consists of three trials with 10-minute intervals between each trial. This challenges animals to go forward 5 turns along the turning path for 60 seconds. Each animal is required to remain on the rotating bar at a speed of 5 rpm for 60 seconds before continuing the test. The test procedure is to record the time the animals stay on a rotating rod at a speed of 4–40 rpm over a period of 300 seconds. A trial ends when the animal either falls off the bar or holds onto the bar and completes a full passive rotation. The test is terminated after 3 consecutive trials (with 15-minute intervals) are completed. After the third trial, the animals are weighed. The latency in seconds for each animal to fall off the rotating rod is analyzed20.
Behavioral change assessment will be conducted using an open field exploratory test. This is widely used to evaluate general locomotor activity and emotional behavior in rodents. The open field is a 100×100 cm test area divided into 25 squares. There are squares called the ‘inner squares’ - the nine squares in the center, the test area, and the ‘outer squares’, which are the 16 adjacent outer squares at the border. Each animal is placed in the central square of the open field 20×20 cm wide. The movements of the rats are videotaped for 5 minutes with the camera placed above the zone. The area is cleaned with a disinfectant solution after each use, washed several times with fresh water and dried. The recorded videos will be examined separately (blindly) by two experts who will analyze and record exploratory behavioral responses from three perspectives: 1) spatial orientation activity expressed as the number of breeding acts; 2) locomotor activity expressed as number of transitions between inner and outer squares and delay to leave the yard; and 3) the emotional status associated with the state of rat stress levels during the experiment, quantified by the number of brush operations and the number of boluses. If there are differences between the two experts, the videos will be reviewed again by each and they will collaborate to reach a consensus20.
The elevated plus maze test assesses rodent anxiety. The device used for testing is a cruciform structure with two open arms and 2 closed dark arms connected to a central platform. The arm is 50 cm from the ground and 90 cm long from the center. The animal is placed in the center of the device and its movements are recorded with a video camera. Test time is 5 minutes for each animal. After each animal experiment, the area will be cleaned once with a disinfectant solution and rinsed several times with fresh water and dried. Video recordings will be analyzed in a blinded manner individually by two different investigators. They will evaluate the individual stress level by registering the number of seconds spent by the animal in closed sleeves and the number of grooming actions. The exploratory activity is evaluated by recording: 1) the number of seconds spent in the open sleeve, 2) the number of seconds spent in the central area, 3) the number of rearings to evaluate the search phase of exploratory behavior, and 4) number of bending over the edge. If there are differences between the two experts, the videos will be reviewed again by each, followed by discussion to reach a consensus score20.
Histopathological evaluation
Organ samples for histopathological analysis (Table 1) will be fixed for 24 hours in 4% PFA (paraformaldehyde) solution. These will then be dehydrated in 3 steps with ethanol solution (70% for 1 hour, 90% for 1 hour, and 100% for 5 hours). Tissue samples will then be embedded in paraffin after a final xylene clearing step of 2 hours. Serial sections of approximately 25 μm thickness will be cut using a microtome and stained with hematoxylin/eosin according to a standard protocol for tissue analysis. The staining protocol consists of extraction of paraffin and rehydration in decreasing ethanol concentration solutions finishing with deionized water, followed by a 3-minute staining step with hematoxylin in glacial acetic acid, then washing in tap water for 5 min and finally destaining in ethanol. Following this, sections are stained with eosin for 30 seconds before being dehydrated with ethanol (95% ethanol 3 times, 100% ethanol 3 times). Finally, sections are cleared with xylene in three 15-minute steps and covered with xylene-based medium. Light microscopy will be used for histological evaluation.
Genotoxicity evaluation
Among the many different types of DNA damage, 8-hydroxydeoxyguanosine (8-OHdG) is a common marker of oxidative DNA damage. 8-OHdG, a by-product of oxidative DNA damage, is formed physiologically and enhanced by chemical carcinogens. DNA will be extracted with Qiagen DNeasy blood and tissue kits, and the levels of 8-OHdG will be determined using an ELISA assay according to manufacturer’s instructions.
The micronuclei assay is another test used to evaluate the genotoxicity of chemicals. Thus, femoral bone marrow cells will be subjected to the micronuclei assay as follows. Cells are suspended in fetal bovine serum and dropped on microscope slides (2 slides per animal), dried, fixed, and stained with the Leucodif 200 kit (Erba Lachema s.r.o., CZ). All slides are coded and examined under light microscopy (Nikon Eclipse Ci-L, Japan). A total of 500 erythrocytes will be counted to determine the proportion of polychromatic erythrocytes (PCEs) among the total of PCEs and normo-chromatic erythrocytes (PCEs + NCEs). The incidence of micronucleated PCE is determined by counting 4000 PCEs per animal.
Oxidative stress evaluation
Potential perturbations to blood and tissue redox homeostasis (including liver, spleen, brain, intestine, pancreas, heart, kidney, and muscle) resulting from exposure to the pesticides will be assessed by measurement of specific redox biomarkers, concerning both antioxidant defence and oxidative damage. Specifically, oxidative modification of proteins will be determined by the estimation of protein carbonyl content (CARBs), through the evaluation of 2,4-dinitrophenylhydrazone (DNP-hydrazone), as produced by the reaction of protein carbonyls with 2,4-dinitrophenylhydrazine (DNPH). Lipid peroxidation will be assessed by evaluating thiobarbituric acid reactive substances (TBARS), such as malondialdehyde (MDA)21 .
The determination of antioxidant parameters will be performed by measuring the most abundant intracellular antioxidant, the reduced form of glutathione (GSH), by reaction with 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) and the formation of 2-nitro-5-thiobenzoic acid (TNB)21,22. Moreover, total antioxidant capacity (TAC), an assay based on the reduction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), will show the cumulative action of antioxidant components present in the biological samples23.
An increase of H2O2 levels, one of the most significant endogenous reactive oxygen species (ROS), is indicative of a temporal redox imbalance24. Catalase is considered to be in the first line of antioxidant defence mechanisms degrading H2O2 into oxygen and water, especially in erythrocytes, due to its abundance together with glutathione peroxidase (GPx). Furthermore, in this biological process inside the cell, peroxiredoxins contribute to H2O2 detoxification25. In addition, glutathione reductase (GR), involved in the glutathione redox cycle, will be measured21,22.
The assessment of the aforementioned redox biomarkers through spectrophotometric methods could provide useful information about the scavenging of free radicals and the overall redox alterations in response to xenobiotic substances26. Previously, other studies also examined the effects of chemical mixture administration on rats by measuring these redox parameters, unveiling the activation of redox adaptations in blood and tissues27,28.
CONCLUSIONS
Our investigation aims to explore possible mechanisms of toxicity and their associated physiological changes caused by exposure to glyphosate or its combination with dicamba and 2,4-D at regulatory relevant doses (ADI, NOAEL). As regulatory agencies currently rely on animal testing for determining the degree of toxicity of chemical pollutants, we anticipate that the results from the GlyphoMix investigation will inform regulators to establish more appropriate ADI values for the herbicides under study in this project, which may currently be too high. In addition, the discovery of any new mechanisms of toxicity from the GlyphoMix study will inform environmental epidemiology investigations in order to test whether exposure to environmental levels of pesticides in ‘real-life’ scenarios can be associated with changes in the levels of biomarkers, which could point to the development of chronic disease. Furthermore, although toxicity tests on laboratory animals are the first choice to assess the adverse effects of chemicals causing human diseases, they do not always reflect human physiology and experimental settings, and do not reflect exposure to multiple chemical toxicants at low environmental levels during sensitive periods of development. A combination of the findings from laboratory toxicity studies, such as the GlyphoMix project presented here, with environmental epidemiology studies, will contribute to more appropriate regulations governing the use of pesticides to better protect public health.