INTRODUCTION
While inflammation may be a physiological defence response, chronic inflammation results as an impairment of the normal process leading to the destruction of normal connective tissue due to an increased enzymatic catalytic activity and cytokines expression1,2. The high rate of the anti-inflammatory diseases around the world (especially rheumatoid arthritis and osteoarthritis) represents the golden topic that scientist have to manage. That is why it is mandatory to develop new anti-inflammatory drugs, characterized by a high rate of efficiency and with many possible side effects3.
Transitions metals are well known and utilized due to their anti-inflammatory and anti-arthritic properties4 since 1962, when several other gold compounds like sodium aurothiomalate, aurothioglucose and sodium aurothiopropanol demonstrated an excellent reliance in severe cases of rheumatoid arthritis.
Due to the last advances in inorganic chemistry, the development of transition metal complexes with organic ligands have gained broad interest, especially for their potential as therapeutic agents. These complexes provide a variety of effects. They were mentioned for their beneficial action in cancer and leukemia since the sixteenth century. The anti-tumor activity of an inorganic complex cis-diammine-dichloroplatinium (II) or cisplatin was discovered and from that moment it has become one of the most widely consumed and effective cytostatic drug for the treatment of solid carcinomas5-8. But platinum is not the singular metal studied for the treatment of cancer, many other transitions metals have been used as anticancer agents. Titanocene dichloride (a complex with titanium) had been developed and acknowledged as active anticancer drug against breast and gastro-intestinal carcinomas9. Gold complexes (e.g. 2-[(dimethylamino) methyl] phenyl gold (III) complex) were studied for their anti-tumor potential, as they act by a mechanism involving the mitochondria as target site10,11. Lanthanum has also been studied for its anti-cancer properties12. Ruthenium complexes which comprise ruthenium with oxidation state +2 or +3 showed anti-proliferative effects in human ovarian cancer cell lines13, ruthenocene derivatives exhibiting pronounced anti-tumor activity, better than hydroxyl tamoxifen14-17, by forming mono-functional adducts with guanine bases on the MCF-7 (ER-positive) breast cancer cell lines18. The ferrocifenes derivatives, having a great affinity for the estrogen receptor, display anti-proliferative properties against hormone dependent and hormone independent breast cancers7,19,20. Other complexes have been used such as: 1) anti-infectious agents like organometallic complexes of Pt, Rh, Ir, Pd and Os, described for their trypanocidal activity21,22 and manganese complexes studied against pathogenic fungi (Collectatrichum capsici and Macrophomina phaseolina) and bacteria (Pseudomonas cepacicola and Klebsella aerogenous)23; 2) antidiabetic agents such as vanadium compounds that are known for their involvement in enabling many insulin-signaling pathways that involve the mitogen-activated-protein kinases (MAPKs), extracellular signal-regulated kinase 1/2 (ERK1/2) and p38MAPK, and phosphatidylinositol 3-kinase (PI3-K)/protein kinase B (PKB)24; and 3) free radical scavengers, such as manganese(II) complexes with bis (cyclohexylpyridine) substituted macrocyclic ligand have been established as a functional copy of superoxide dismutase25.
The ability of this transition metals to react with different ligands enabled the development of a series of complexes with different pharmacological action, in most cases superior to the original ligand action3,26. Piroxicam may proceed as a multiple-site ligand and several designs engaging its bonding to different metal ions can be analyzed. By operating as chelate ligands with coordination involving the enolate oxygen atom and the oxygen atom of the amido group, metal complexes with other therapeutic agents of the same class have been recently reported in the literature. In this regard, complexes of Co(III), Ni(II), Cu(II) and Zn(II) have anti-inflammatory action above N,N’ - propylenebis-salicylamide27, enhancing again that the complexes’ possible effects are superior to that of the single ligand, especially for the Co(II), Cu(II) and Zn(II) complexes. Co(II), Cu(II)28 and Zn(II)29,30 complexes of many non-steroidal anti-inflammatory drugs have been shown to exhibit pronounced anti-inflammatory effects, when solely the ligand is simply deemed to be a carrier that leads the metal to the therapeutic target. Studying the inflammation process, higher concentration of zinc ions in inflamed tissues were confirmed. This emphases the idea that this transition metal has anti-inflammatory properties31.
Currently, the oxicams complexes with transition metals as the active ingredient are considered a group of particular interest in the design of new drugs products, including topical formulations, with superior pharmacological action and without side effects at the level of the gastro-intestinal tract32-34. Previous studies concerning the transfer of oxicams from their complexes with Cu(II) were carried out using acrylamide based polymer hydrogels35.
The acute inflammatory response is well known as an acute increase of vascular permeability and cellular infiltration, which causes edema, also as a result of fluids and proteins extravasation and leukocyte accumulation. Edema is one of the defining clinical signs of local inflammation and its measurement in the plantar paw of mice/rats can indicate the inflammatory or anti-inflammatory effects36.
Since the development of nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors, carrageenan-induced inflammation in the rat paw became the classical widely performed model of edema formation and hyperalgesia37. More recently, COX-2 was shown to reach maximal expression 1 hour after the local carrageenan injection38,39. In the literature there are about 400 studies that describe this model. Among these, it can be acknowledged that there are particularly three studies that are widely quoted, those of Levy40, Sugishita et al.41, Henriques et al. 42. Levy40 concluded that by using an injection of 1% carrageenan in the mouse paw, the obtained edema is similar to the rat, as time course, but less potent in proportion. Sugishita et al. 41 described in more detail the mouse paw edema, in the acute form, using 3% carrageenan. Henriques et al.42 presented a biphasic carrageenan-induced edema into the mouse paw. The first phase of the inflammatory process is due to a low-intensity edema, and it is carrageenan-dose independent, while after 24 h the second phase evolves, exhibiting a more marked edema with a maximum effect between 48 and 72 h. Lately, it has been shown that 1% carrageenan induces a marked powerful edema in BALB/c mice, but in this case only the second phase has been proven43. At present, the most widely used methods are those described by Levy40 and Sugishita et al.41. Nowadays, the carrageenan injection administered to the hind paw of rats remains the common model for studying inflammation and inflammatory pain due to its simplicity: carrageenan causes edema, an increase in the paw volume, and an exacerbated sensitivity and mechanical stimuli which are known as hyperalgesia. Conventional non-steroid anti-inflammatory drugs (NSAIDs), COX-2 inhibitors and prostaglandin E2 (PGE2) monoclonal antibodies are effective anti-inflammatory agents in these models. In the paw edema model, COX-2 levels were found elevated with a concomitant increase in prostaglandin production44.
Using this raw data, we analyzed the anti-inflammatory effect of Co(II) complexes with oxicams on the inhibition of hind paw edema induced in rats, using a carrageenan solution as a positive control and a digital plethysmometer.
METHODS
Animal model
In this study, 48 Wistar rats of both sexes, kindly provided by the Cantacuzino Institute Biobase, with health certificate, were used. The average weight of the rat cohort was 150 ± 10 g. The rats were retained in standard laboratory conditions (22 ± 2 °C room temperature and 40–50% relative humidity) with 12 h light and dark cycles, and were fed and watered ad libitum. Prior to the experiment, the animals were fed pellet food and acclimatized to laboratory conditions for 2 days. The cohort was split into equal groups of 6 animals, homogeneous for each test sample and control groups. All experiments were performed according to the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. The ARRIVE guidelines45 for reporting experiments using live animals were acknowledged and the experiments were approved by the Ethics Committee of the ‘Carol Davila’ University of Medicine and Pharmacy Bucharest, Romania Nr. 230/07.01.2011.
Sample formulations for administration
This study used semisolid formulations, emulsion-gel type, with polyacrylate as hydrophilic macromolecular compounds, for local use, containing cobalt complex with anti-inflammatory nonsteroidal agents from oxicams group (0.01–10%).
Combining the hydrophilic matrix with the non-ionic agent and the lipophilic absorption promoters as isopropyl-myristate, with or without compatible lipidic compounds, generates biphasic semisolid systems, with a minimum volatile alcohol content. The formulations contain also a non-ionic solubilization agent (1–5%), in order to maintain the active substance in solution or to stabilize the dispersion and lipophilic absorption promoters isopropyl-myristate type (1–10%), in the presence of a small quantity of alcohol (5–20%).
The two substances (salts of the oxicam, i.e. meloxicam or piroxicam, with cobalt), alone or in combination, have been embedded in a hydrophilic matrix in the form of aqueous dispersions, co-solvent system including ethanol and at least one promoter of absorption (Table 1). The trainer network has been hydrated for 24 hours in the form of a 2% dispersion of the carbomer polyacrylic 940. For this system, the gel structure was carried out by triethanolamine neutralization. Co-solvents including active entities were dispersed in the bulk hydrated gel and homogenized for 10 minutes at 2000 rpm (using a mechanical stirrer, Heidolph RZR 2020 Germany, equipped with turbine and container with useful volume of 50 mL and round bottom). Individual quantities of 20 g gel were prepared. The compounds were individually weighed with an analytical balance type Kern (accurately 0.1 mg), except the active substance (0.05, 0.1 and approximately 0.29%), for which was used a semi-micro analytical balance (Radwag XA 210 / X, Radwag Wagi Elektroniczne, accuracy: 0.01 mg).
Table 1
Qualitative and quantitative composition of semisolid topic formulations, with oxicams salts (meloxicam or piroxicam) and cobalt
The materials were as follows:
Fancol® Iso, (isopropyl myristate, lanolin, oleyl alcohol), HSH Chemie, Germany.
Cremophor® EL, (PEG-35 Castor oil, polyoxyl 35 Castor oil), BASF SE 67056 Ludwigshafen, Germany.
Triethanolamine >99% purity, Carl Roth GmbH + Co. KG, Germany.
Propilenglycol, Sigma Aldrich, USA.
Ethanol absolute, Chromasolv, lot 91240, Sigma Aldrich, USA.
Isopropyl myristate, Scharlau Chemie S.A., Spain.
Purified water (conductivity under 0.05 µS/cm) was obtained with a SGW Ultraclear UV PlusTM system.
According to Table 1, the following gels formulations were tested:
Meloxicam Co 0.1% (isopropyl myristate), encoded MxCo 0.1% (IPM);
Meloxicam Co 0.05% (isopropyl myristate, lanolin, oleyl alcohol), encoded Mx-Co 0.05% (ISO);
Meloxicam Co 0.56% (isopropyl myristate, lanolin, oleyl alcohol), encoded Mx-Co 0.56% (ISO);
Piroxicam Co 0.05% (isopropyl myristate, lanolin, oleyl alcohol), encoded Px-Co 0.05% (ISO); and
Piroxicam Co 0.05% (isopropyl myristate), encoded PxCo 0.05% (IPM).
Two commercially topical products were used as reference:
Experimental design
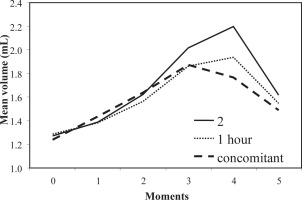
In order to determine the optimal interval between the time of treatment and the administration of the pro-inflammatory agent, three doses of the reference sample at different time intervals were made, before the phlogistic agent administration (immediately, one hour, two hours). After the results (Figure 1), a management decision to use the first administration diagram was taken.
Figure 1
Anti-inflammatory action of semisolid formulations for organometallic complexes containing cobalt combinations with oxicams at 2 h, 1 h and concomitant with edema inducement
The topical tested formulations and reference substances were administered subcutaneously, about 5 minutes before the positive control. Subsequently, 0.1 mL saline solution of 1% carrageenan was injected to the right hind limb (from seaweed, Sigma Aldrich, USA).
A quantity of 4 g of the test semisolid formulations and the reference gels were spread uniformly by massaging repeatedly for 50 times in order to facilitate absorption through the skin of the right face of the animal’s foot. For the control group, the inflammatory agent was applied without any another treatment.
The right hind limb volume was measured prior to the treatment with the phlogistic agent; therefore, we represented the initial time of all the measurements, and immediately after the injection, then at 60, 120, 180 minutes and 24 hours.
In order to register readings, the inflamed part of the lateral malleolus was end-labeled and then the immersion liquid was introduced into the measuring cell of the device up to this level. Four measurements for each animal and for each moment established by the working protocol were performed. An Ugo Basile 7140 - Italy plethysmometer and a CUB software program were used for data acquisition and storage.
Statistical analysis
The experimental data were expressed as mean ± standard deviation and analyzed by one-way analysis of variance (ANOVA); t-test was used to test significance. The results were considered statistically significant for p≤0.05. All statistical tests were carried out using SPSS statistical software.
RESULTS
The mean values of the measured volumes for each animal and the time of measurement, mean values and standard deviation group were calculated. The increased volume of the inflamed limb at each measurement time was expressed as a percentage compared to the initial volume (at t0). The anti-inflammatory effect was evaluated by calculating the percentage changes between average values in treated groups and control groups (with or without treatment), for each measurement point.
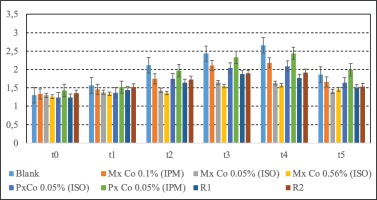
The average values of the extremities volumes (mL) and standard deviation before the treatment and the injection with the phlogistic agent (at t0), immediately after treatment and injection (at t1), at 60 minutes (t2), 120 minutes (t3), 180 minutes (t4) and 24 hours (t5), are represented in Table 2 and Figure 2.
Table 2
The means and standard deviations of extremities volumes at different time points
| Lots | Measurement times | |||||
|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t3 | t4 | t5 | |
| Blank | 1.30 ± 0.08 | 1.57 ± 0.01 | 2.12 ± 0.08 | 2.43 ± 0.22 | 2.66 ± 0.01 | 1.86 ± 0.06 |
| MxCo 0.1% (IPM) | 1.33 ± 0.02 | 1.46 ± 0.07 | 1.75 ± 0.18 | 2.11 ± 0.05 | 2.18 ± 0.13*** | 1.66 ± 0.12 |
| MxCo 0.05% (ISO) | 1.30 ± 0.10 | 1.38 ± 0.18 | 1.43 ± 0.18 | 1.65 ± 0.16 | 1.63 ± 0.16*** | 1.40 ± 0.07** |
| MxCo 0.56% (ISO) | 1.26 ± 0.14 | 1.34 ± 0.19 | 1.36 ± 0.24 | 1.55 ± 0.17 | 1.57 ± 0.18*** | 1.46 ± 0.22** |
| PxCo 0.05%(ISO) | 1.24 ± 0.02 | 1.37 ± 0.06 | 1.75 ± 0.08 | 2.04 ± 0.04 | 2.09 ± 0.12*** | 1.64 ± 0.04 |
| PxCo 0.05% (IPM) | 1.43 ± 0.04 | 1.52 ± 0.11 | 1.97 ± 0.22 | 2.33 ± 0.12 | 2.44 ± 0.13 | 1.99 ± 0.15 |
| R1 | 1.24 ± 0.07 | 1.44 ± 0.05 | 1.64 ± 0.06 | 1.87 ± 0.15 | 1.77 ± 0.17 | 1.49 ± 0.13 |
| R2 | 1.35 ± 0.05 | 1.52 ± 0.22 | 1.73 ± 0.12 | 1.89 ± 0.01 | 1.92 ± 0.08 | 1.53 ± 0.03 |
Figure 2
Mean volumes (mL) measured with the plethysmometer for the tested gels. Data presented as mean ± S.E.M (N=6)
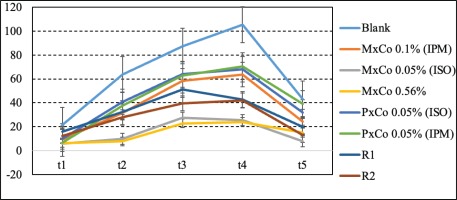
The percent changes in volume were calculated at each time point compared to baseline (Table 3 and Figure 3), using the following formula:
Table 3
Percent change in volume at each time point compared to baseline
Figure 3
Volume variations (%) compared to the initial time measurement. Data presented as mean ± S.E.M (N=6)
Percent change in volume = (Vt - V0)/V0 ×100,
where V0 is the initial volume of the extremity, and Vt is the volume measured at each time point.
The anti-inflammatory effect in percentage was calculated by using the formula:
Percent anti-inflammatory effect = 100 × [1 - (a-x)/(b-y)], where a is the average volume of the treated limb (mL), b is the average volume of the untreated control rats (mL), x is the average of the initial volume of the treated limb (mL), and y is the average of the initial volume of the end to untreated control rats (mL).
The anti-inflammatory percentage effects at the measurements time are presented in Table 4 and Figure 4. Values of the anti-inflammatory effect below 20% were considered as proof of lack of activity against the phlogistic agent46. In the control group, receiving the phlogistic agent without further treatment, the inflammatory response, apparent from the first measurement, reached a maximum at 3 hours (t4) and was maintained high at 25 hours (t5). One hour after inducing the edema, the anti-inflammatory effect varied between 34.53% and 88.07% for all tested gels. PxCo 0.05% (ISO) and PxCo 0.05% (IPM) had a comparable anti-inflammatory effect of between 34–39%. MxCo 0.05% (ISO) and MxCo 0.56% (ISO) displayed the most pronounced anti-inflammatory effect, at 88% and 84%, respectively.
Table 4
Percent anti-inflammatory effect obtained at different time points after injection
Figure 4
Percent anti-inflammatory effect for meloxicam and piroxicam combined with the metal solely. Data presented as mean ± S.E.M (N=6)
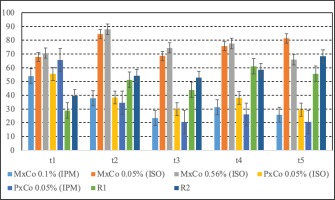
The anti-inflammatory effect of the tested samples decreased after 120 minutes, but was still above the threshold value. Accordingly, MxCo 0.05% (ISO) and MxCo 0.56% (ISO) were between 68–74%, whereas samples with PxCo 0.05% (ISO), PxCo 0.05% (IPM) and MxCo 0.1% (IPM) were close to the lower limit at 29.95%, 20.68% and 23.4%, respectively.
After 180 minutes, the two samples of piroxicam complexes had an anti-inflammatory effect >20%. The most pronounced anti-inflammatory effect was assigned to MxCo 0.56% (ISO) at 78%, followed by MxCo 0.05% (ISO) with 75% and MxCo 0.1% (IPM) at 31.17%.
At 24 hours after administration of the phlogistic agent, both samples, PxCo 0.05% and MxCo 0.1% (IPM), retained their anti-inflammatory effect, but at a lower level, while MxCo 0.05% (ISO) and 0.56% (ISO) had a greater anti-inflammatory effect (>60%).
DISCUSSION
Limitations
There are a few limitations of the study. Firstly, strong and prolonged anti-inflammatory effect upon single application is translated into the normalization of the rat paw thickness after 24 h of the carrageenan injection. Taking into account that the main mechanism explained for the inflammation process refers to the inhibition of cyclooxygenase and prostaglandin release, a more established analgesic action is to be considered for longer duration upon single application compared to the reference gel. This is correlated with a dosing program of maybe two or three times administrations daily, for long-drawn anti-inflammatory and analgesic effects.
CONCLUSIONS
After testing samples of some transition metal complexes with oxicams ligands for the possible anti-inflammatory activity in vivo (rat paw edema model), it was concluded that PxCo 0.05% (ISO), has a relatively low, but constant effect during measurements. The most pronounced anti-inflammatory effect maintained throughout the experiment was observed for MxCo 0.56% (ISO), with values between 70.68% and 88.07%, followed by MxCo 0.05% (ISO) between 67.86% and 84.46%. MxCo 0.05% (ISO) and MxCo 0.56% (ISO) samples have displayed an anti-inflammatory effect, higher compared to the reference samples at all moments of evaluation. Further efforts should also be made towards new strategies for the delivery of metal-based drugs in physiological media and in vivo, aiming to reduce the undesired side effects.


