INTRODUCTION
Cholinesterase is a family of enzymes that catalyzes the hydrolysis of the neurotransmitter acetylcholine into choline and acetic acid, a reaction necessary to allow a cholinergic neuron to return to its resting state after activation. It involves two types of enzymes: 1) acetylcholinesterase or erythrocyte cholinesterase (E.C. 3.1.1.7); and 2) butyrylcholinesterase (EC 3.1.1.8), also known as plasma cholinesterase or butyrylcholinesterase1-3.
Acetylcholinesterase is found in many types of conducting tissue: nerve and muscle, central and peripheral tissues, motor and sensory fibers, and cholinergic and non-cholinergic fibers. Acetylcholinesterase is also found in the red blood cell membranes, where it constitutes the Yt blood group antigen. Butyrylcholinesterase is found primarily in the liver and plasma which hydrolyzes butyrylcholine more quickly than AChE4.
Organophosphate pesticides are known potent inhibitors of AChE, present in the central and peripheral nervous system, which breaks down the neurotransmitter acetylcholine leading to the clinical signs and symptoms of organophosphate poisoning5,6. They are among the most widely used pesticides in the Asian countries and are used in both agricultural and residential settings. Most OP-pesticides have the same general structure, a common mode of action as an insecticide, and a common mode of acute toxicity. The measurement of BChE and AChE activities are used widely to investigate incidents of OP over-exposure, or as part of health surveillance in workers at risk of OP exposure. It can also be used for diagnosis after potential exposure to nerve agents and verification of treatment effectiveness, e.g. for Alzheimer’s disease therapy6,7.
Acetylcholinesterase activity in human blood reflects the status of synaptic AChE which is the target enzyme of anticholinesterases8-10. However, most organophosphorus compounds inhibit BChE faster than AChE, and the inhibition of BChE is therefore a more sensitive indicator of the absorption of these agents. Moreover, the activities of AChE have been found to correlate well with the clinical status of OP-poisoned patients, as against BChE and their patterns of inhibition vary with the type of OP-pesticide11-13. Consequently, more information about the exposure of an individual to anticholinesterases can be obtained if both AChE and BChE activities are determined. The procedure for determination of BChE activity in human plasma or serum is well established and used frequently, while this is not the case with the erythrocyte AChE. A great variety of methods have been described in the past for cholinesterase activities14,15. A widely used method for determining cholinesterase activities is the spectrophotometric method of Ellman et al.16 with thiocholine esters as substrates and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) as the thiol reagent. It is based on the reaction between thiocholine, which is produced by the enzymatic hydrolysis of the synthetic substrate ATCh and DTNB or Ellman’s reagent. The formation of the yellow product of this reaction, 5-thio-2-nitrobenzoic acid (TNB), is measured by monitoring absorbance at 412 nm. The Ellman method has repeatedly been modified in order to meet requirements for different applications. A number of studies have reported the use of different inhibitors for the simultaneous measurement of AChE and BChE activities. Meuling et al.17 used propionyl thiocholine as substrate, and measured the enzyme activity in the presence and absence of the selective BChE inhibitor, ethopropazine (ET) by recording the absorbances of the reaction product at 415 nm and 450 nm. The activities of AChE and BChE were calculated from the differences between activities measured in the presence and absence of ET. These measurements require an instrument that can simultaneously measure the absorbance at two wavelengths. Reiner and Simeon-Rudolf18 suggested ATCh and butyryl thiocholine (BTCh) as substrates to determine AChE and BChE activities in whole blood, respectively. Worek et al.19 suggested a method for determining AChE activities in whole blood by measuring the activity at 436 nm with ATCh by inhibiting BChE by ET and BChE was measured separately using BTCh. The measurement at 436 nm, reduced the indicator absorption to 80% and the hemoglobin absorption to 25% compared to the usual Ellman procedure with 412 nm wavelength. Similarly, Naik et al.20 used ET for BChE inhibition for measuring AChE activities in whole blood from humans as well as experimental animals.
Thus, simpler and direct procedures are very much desirable for measuring BChE and AChE activities in blood, which can be useful for routine toxicologic or clinical work. In this study, a methodological approach is proposed with procedures for determining AChE and BChE based on Ellman’s colorimetric method, which uses the same substrate (ATCh), color reagent, and buffer solutions both for BChE and AChE activity.
METHODOLOGICAL APPROACH
Chemicals
Acetylthiocholine iodide, 5,5’-dithio-bis-2-nitrobenzoic acid were from Sigma, disodium hydrogen phosphate (Na2HPO4.2H2O), potassium dihydrogen phosphate (KH2PO4), sodium chloride and all other chemicals were from Merck.
Color reagent
To 500 mL phosphate buffer (pH 7.2; 26 mmol/L) are added 51 mg DTNB and mixed to dissolve and stored in Ambercolored bottle at 2–8℃ (stability 1 month).
Acetylthiocholine iodide solution (substrate reagent)
A volume 2 mL distilled water was added to the glass vial containing pre-weighed 90.2 mg ATCh, mixed to dissolve and stored at 2–8℃ (stability 2 weeks).
Thiocholine solution
Obtained from chemical degradation of acetylthiocholine iodide. A solution of 15 mmol/L acetylthiocholine, pH 10 is incubated at 37℃ for 5 h21. The resultant thiocholine solution is neutralized to pH 7.2 and diluted with distilled water before use to desired concentrations for the linearity study (both stock and working standards to be prepared fresh).
Normal saline
To 100 mL distilled water is added 0.9 g sodium chloride, mixed to dissolve and stored at 2–8℃ (stability 1 month).
Control plasma
Commercially available control plasma samples were purchased from Prathama Blood Centre, C. V. Raman Marg, Vasna, Ahmedabad-380007, Gujarat, India.
Procedures
Blood samples (2–3 mL) were collected from antecubital vein in vacutainer tubes containing EDTA for the analysis of cholinesterase activity from normal donors (n=8). Prior written consent was taken from the subjects.
Thoroughly mixed (by inversion) aliquot (0.1 mL) of each blood sample was diluted 25-fold with normal saline (saline diluted blood) and used for determination of AChE activities while the remaining blood was centrifuged to separate plasma from erythrocytes and used for the BChE activities. The activities of AChE and BChE were measured at 412 nm in phosphate buffer (pH 7.2, 26 mmol/L) with DTNB as thiol reagent and ATCh as substrate by modified spectrophotometric method of Ellman et al.16. The method was standardized by using both primary (thiocholine) and secondary (control plasma) standards.
For AChE activities, 3 mL of color reagent was added to two clean glass tubes each, labeled ‘Tube-T’ and ‘Tube-P’. After mixing thoroughly, 0.1 mL of ‘saline diluted blood’ was transferred to ‘Tube-T’. The remaining diluted blood was centrifuged at 2000 rpm for 7 min at room temperature and 0.1 mL of the supernatant was added to ‘Tube-P’. The increase in absorbance per minute was read for both the tubes at 412 nm at 37℃ separately on UV-Visible spectrophotometer (Model UV-1800, Shimadzu, Japan) under kinetic mode immediately after addition of the substrate. The value of AChE activity was obtained after subtracting the value of ‘Tube-P’ from ‘Tube-T’.
The BChE activity in plasma was also measured with the same concentration of buffer, DTNB and ATCh as the AChE activity measurement. The BChE activity values are corrected by subtracting corresponding blank values without substrate addition. The exact procedures for AChE and BChE are given in Table 1.
Table 1
Standard procedure for determination of acetylcholinesterase and butyrylcholinesterase activities
During the enzyme assay, the final dilution of blood for AChE activities was 780-fold and that of plasma for BChE 156-fold. The activities of AChE and BChE were expressed as micromoles of ATCh hydrolyzed per minute per liter (i.e. Units/L) based on the molar absorption coefficient for the dianion of DTNB at pH 7.2.
Calculations
Enzyme activities of AChE, BChE were calculated using the following equations:
Activity of AChE (Units/L) = ΔA × 56520
Activity of BChE (Units/L) = ΔA × 11300.
They are derived from the following general equation:
Activity of Enzyme (AChE, BChE) in µmol ATCh hydrolyzed/min/L (Units/L)
= [ΔA/1.38×104] × [3.12/V] × 106
where ΔA is the increase in absorbance/min; 3.12 is total assay volume in mL; 1.38×104 is the molar absorption coefficient (M-1cm-1) for the dianion of DTNB at pH 7.422; and V is the sample volume of plasma (0.02 mL) for BChE or blood (0.004 mL) for AChE used in the assay.
Color reagent stability
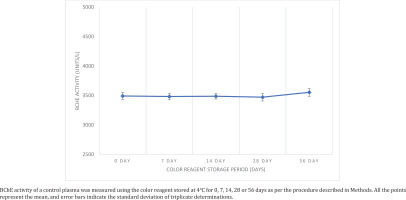
A commercial control plasma sample was divided into five aliquots and stored separately at -20℃ for 0, 7, 14, 28 and 56 days. Freshly prepared color reagent was separated into about 10 mL aliquots (n=4) and stored separately at 4℃ in the dark, to be used for analysis of the plasma aliquots. Freshly thawed plasma aliquots were assessed at 7, 14, 28 and 56 days of color reagent storage to observe decomposition of DTNB by measuring BChE activity (in triplicate) and results were compared with those obtained with fresh color reagent.
Effect of phosphate buffer concentration
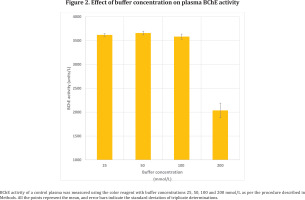
A commercial control plasma was separated in 3 aliquots and assayed as per the proposed procedure using the color reagent with phosphate buffer of 4 different concentrations (25, 50, 100 and 200 mmol/L).
Linearity of the colorimetric measure
Though the primary standard thiocholine is not generally preferred in the assay due to its unstable property, the linearity was checked both by using thiocholine and control plasma.
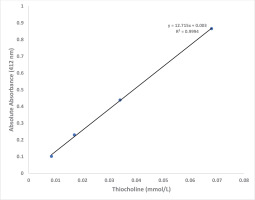
For linearity with a thiocholine concentrations, dilute solutions bearing different concentrations (0.125, 0.25, 0.5 and 1.0 mmol/L) of thiocholine were prepared by diluting indicated stock thiocholine solution with distilled water. A 0.20 mL of dilute preparation was added to 2.90 mL of color reagent, the mixture incubated at 37℃ for 10 min and the absorbance was read at 412 nm on spectrophotometer against reagent blank.
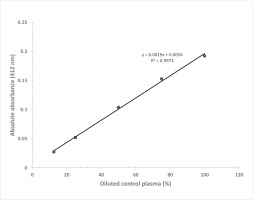
Linearity of the colorimetric measure with control plasma was performed by using a commercially available control plasma sample. It was diluted to 100, 75, 50, 25 and 12.5% in normal saline and analyzed in duplicates for BChE values using the proposed assay procedure.
Validation of assay
Intra-day precision
Two of eight normal fresh blood samples were used for validation. For intra-day precision, each of these blood samples was analyzed on the same day for five repetitive measurements for AChE activity in duplicates by using the proposed method. Plasma was separated from these blood samples and divided in to ten aliquots each and five aliquots of each sample were analyzed for repetitive measurements for BChE activity in duplicates on the same day while the remaining plasma aliquots (five each) were stored frozen (-20℃) for inter-day precision.
Inter-day precision
Freshly thawed aliquots (n=2) of plasma samples were assayed for BChE activity (in duplicates) by using the proposed method for five consecutive days. Inter-day precision was not performed for AChE activity since it has to be performed from fresh blood. Mean, standard deviation and co-efficient of variation (CV%) were calculated from average values of duplicate measurements.
Comparison with the kinetic Ellman method
For comparison, analysis of control plasma samples (n=8) from normal individuals for BChE activity was performed by the proposed method and the original kinetic procedure of Ellman et al16.
A typical run of the kinetic Ellman method for BChE used 3 mL pH 8.0 phosphate buffer, 100 μL 10 mmol/L DTNB in pH 7.0 phosphate buffer, and 20 μL plasma. After 5 min of incubation at 25℃, 100 μL of 15 mmol/L ATCh solution in distilled water was added as the substrate and the increase in absorbance per minute was read at 412 nm on UV-Visible spectrophotometer under kinetic mode. The activities of BChE were expressed as micromoles of ATCh hydrolyzed per minute per liter (i.e. Units/L) based on the molar absorption coefficient for the dianion of DTNB at pH 8.0 (1.36×104 M-1cm-1)16.
RESULTS
Color reagent stability
We observed no significant change in BChE activity values of commercial control plasma sample obtained by using color reagent aliquots stored at 4℃ for 7, 14, 28 or 56 days compared to its value with the fresh color reagent (Figure 1). Moreover, there was negligible change in the intensity of yellow color of the reagent during the storage period, directly measured at 412 nm, which may increase due to chemical degradation of DTNB to TNB on storage (change in absolute absorbance after 56 days was only 0.003). Thus, the color reagent is quite stable when stored in the dark at 4℃.
Effect of phosphate buffer concentration
The effect of different buffer concentrations of color reagent (25, 50, 100 and 200 mmol/L) on BChE activity is shown in Figure 2. Increase in buffer concentration of the color reagent to 200 mmol/L showed decrease in BChE activity but there was no significant change in the activity from 25 to 100 mmol/L buffer concentrations.
Linearity of the colorimetric measure
Figure 3 shows that the absorbance at 412 nm is directly proportional to the thiocholine concentration in the working range of standards with final concentration in the assay: 0.0085, 0.017, 0.034 and 0.068 mmol/L (R2=0.9994). A linear graph with high correlation (R2=0.9973) was also obtained when BChE activity values were plotted against % dilution of control plasma (Figure 4). Based on our experience, the plasma or serum sample has good stability when stored at -20℃ (more than 6 months) and pooled plasma samples as well as commercially available quality control sera can be used as in-house reference standard for cholinesterase activity after analyzing BChE by the proposed method. Each laboratory should establish its own control reference values for AChE and BChE activities with the proposed assay. The values observed at our center are blood AChE: 2800–5700 U/L and plasma BChE: 1300–3200 U/L.
Precision of assay
Intra-day CV values for AChE activity were 1.20% and 3.29%, with an overall value of 2.25% and for BChE activity the values were 0.74% and 1.64%, with an overall value of 1.19%. Inter-day CV values for BChE activity were 5.17% and 1.28%, with a mean of 3.23% (Table 2).
Table 2
Precision study: plasma butyrylcholinesterase and RBC acetylcholinesterase activities (Units/L) in aliquots of two normal blood samples
Comparison with the kinetic Ellman method
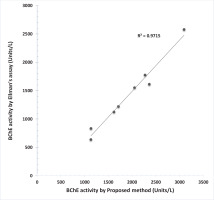
Butyrylcholinesterase activities of plasma samples from normal individuals with the present procedure highly correlated (R2=0.97) with the kinetic Ellman method (Figure 5).
DISCUSSION
A widely used method for determining cholinesterase activities is the spectrophotometric method of Ellman et al.16 with thiocholine esters as substrates and DTNB as the thiol reagent forming the yellow dianion TNB. The method has repeatedly been modified in order to meet requirements for different applications with the use of different thiocholine esters and the phosphate buffer as reaction medium in these assays17,19,23-25. Maximum activity of the enzyme is obtained for pH 8.5–9.0 with butyryl thiocholine substrate at 37°C. An apparent increase in spontaneous hydrolysis of substrate with increasing pH is reported. Based on this the use of phosphate buffer, pH 7.4, was suggested by Silk et al to reduce the undesirable non-enzymic hydrolysis to negligible proportions despite of a pH optimum of 8.526. We preferred to use pH 7.2 to further reduce non enzymic hydrolysis without affecting the linear relationships of the assay procedure at 37°C.Moreover, the effect of temperature on the pH of the phosphate buffer is small therefore the buffer can be prepared at room temperature and used at a temperature ranging 10–40℃. The stability of DTNB in Ellman’s reagent depends on pH of the buffer medium and the temperature; and known to decrease with increased hydroxide concentration (pH). A study by Danehy et al.27 on 0.1 mol/L phosphate buffer at room temperature reported no loss of DTNB in 7 weeks at pH 7.0; 5% loss in 48 h at pH 8.0; 9 % in 4 h at pH 9.3, and complete decomposition within 15 minutes at pH 12.
Procedural comparisons of our proposed method of AChE with different other assays based on DTNB in phosphate buffer with varying concentrations and pH are given in Table 3. Majority of the cholinesterase assays based on Ellman’s reagent using the mixture of buffer and DTNB as color reagent, require freezing temperatures for storage16,23,25. We observed no effect of phosphate buffer concentration from 25 to 100 mmol/L on BChE activity so in the proposed method, we have used the color reagent containing phosphate buffer with 26 mmol/L concentration (pH 7.2) with DTNB 0.26 mmol/L. Our observations show that this reagent is quite stable and can be used at least for two months when stored in the dark at 4℃. At lower concentration of buffer (26 mmol/L), recrystallization of buffer salts does not take place during its storage at 4℃ which is a problem with higher buffer concentrations or freezing temperatures. The present modifications allow the reagents to be handled easily and makes the technique simpler.
Table 3
Comparison of other RBC cholinesterase methods with the proposed acetylcholinesterase assay
| Method | Worek et al.19 | MacQueen et al.23 | Brogdon24 | Reiner et al.25 | Ellman et al.16 | Proposed method |
|---|---|---|---|---|---|---|
| Sample | Hemolyzed washed RBCs | Hemolyzed washed RBCs | Diluted whole blood | Washed RBCs | Diluted whole blood | Diluted whole blood |
| Phosphate buffer (mmol/L) | 100 | 200 | 50 | 100 | 100 | 26 |
| pH | 7.4 | 8.0 | 7.4 | 7.4 | 8.0 | 7.2 |
| DTNB (mmol/L) | 0.32 | 0.30 | 0.13 | 0.30 | 0.08 | 0.26 |
| Inhibitor | ET 20 µM | Not used | Not used | ET 20 µM | QS 0.33 mg% | Not used |
| Incubation time with DTNB before substrate (min) | 10 | Not done | Not done | 10 | Not done | 10 |
| Substrate conc. in incubation mixture (mmol/L) | 0.45 | 9.86 | 1.04 | 1.00 | 0.49 | 4.90 |
| Temperature (℃) | 37 | 37 | 25/37 | 37 | 25 | 37 |
| Wavelength (nm) | 436 | 450 | 410 | 436 | 412 | 412 |
| Mode | Kinetic 3 min | End-point 10 min | End-point 10 min | Kinetic 3 min | Kinetic 6 min | Kinetic 2 min |
| Stopping reagent | No | Quinidine sulphate (QS) | No | No | No | No |
| Units | mU/µmol Hb | ∆pH | µmol ATCh hydrolyzed/min/mL blood | µmol ATCh hydrolyzed/min/mL blood or µmol ATCh hydrolyzed/min/mg Hb | Moles ATCh hydrolyzed / min per RBC | µmol ATCh hydrolyzed/min/L of blood |
In the method of Worek et al.19, AChE activities in whole blood are measured by inhibiting BChE by ET using ATCh as substrate. They measured BChE activities separately in plasma using BTCh as substrate. This determination requires two different substrates, one selective inhibitor, and centrifugation of the whole blood to obtain the plasma. But since ET is known to inhibit AChE and BChE both, correction is required for AChE activity measurements25. Moreover, they suggested the use of 436 nm wavelength to avoid significant hemoglobin absorption while measuring the AChE activities from whole blood samples. It has been reported that a dilution of blood samples over 600-fold is sufficient to prevent the interference by hemoglobin in AChE assay20. We have used the blood dilution of 780-fold in our proposed assay.
Worek et al.19 and MacQueen et al.23 used washed erythrocytes in their AChE assays. Since Reiner et al.28 reported that BChE in residual plasma of unwashed erythrocytes does not affect AChE activity28, the view is that it is not necessary to wash erythrocytes before the AChE assay for clinical purpose, so RBC washing step is not included in the proposed method for simplicity.
DTNB reacts not only with thiocholine, but also with thiol groups in the blood and the reaction between DTNB and thiol groups is completed within 10 min28. Keeping this in mind, we have included 10 min incubation time for the sample with the color reagent before addition of the substrate.
In the procedure of Portmann29, AChE and BChE are both measured in the whole blood. The activity of both the enzymes is first measured with ATCh (1.0 mmol/L) after which BTCh (5.0 mmol/L) is added to the same cuvette and the activity measurement continued. The calculation of AChE and BChE activities is based on the assumption that BTCh is hydrolyzed only by BChE and that the addition of BTCh stops the hydrolysis of ATCh. One should, however, bear in mind that BTCh is not fully specific for BChE. The advantage of this protocol is that it does not require blood centrifugation. The proposed assay involves centrifugation steps for the separation of plasma and for preparation of sample blank, which is a disadvantage that stored blood samples (which are usually hemolyzed) cannot be analyzed.
MacQueen et al.23 have reported the optimal substrate concentrations for erythrocyte and plasma cholinesterase as 2.1 and 8.3 mmol/L ATCh and have used the same buffer-color substrate reagent both for BChE and AChE assays keeping a final concentration of 9.86 mmol/L. Since ATCh is a suitable substrate for both AChE and BChE, and for routine purposes it is convenient to use the same substrate concentration for both enzymes, we have used ATCh (4.9 mmol/L) in both the protocols for the analysis of AChE and BChE activity.
Thiocholine is the choice as a primary standard but it is not stable. Gary and Routh30 used glutathione as a secondary standard; however, this is based upon the assumption of identical molar absorptivity of the sulfhydryl groups of thiocholine and glutathione. On the basis of the high correlation obtained on comparing the results of BChE analysis of 49 normal human sera by the Michel’s ∆pH method with different other methods including the kinetic Ellman method, MacQueen et al.23 observed that these methods of cholinesterase assay gave results within the bounds of clinical requirements and it seemed more appropriate to utilize a reference serum as a secondary standard. Since the plasma or serum sample has good stability for cholinesterase when stored at -20 C, so we propose to use either pooled plasma samples or commercially available quality control sera with pre-determined ChE activities as in-house reference standard for the present assay.
Cholinesterase activities obtained by this proposed method highly correlated with the Ellman method, confirming its accuracy and suggests that it gives results within the bounds of clinical requirements. It can be performed even on a semiautomatic colorimeter (biochemistry/clinical chemistry analyzer) capable of measuring absolute absorbance in kinetic mode with color filter (at 405 nm) that are usually available in a hospital or a clinical/pathological laboratory.
Limitations
The proposed assay is useful to determine both AChE and BChE activities from fresh non-hemolyzed blood samples but it has the disadvantage that blood AChE activity cannot be determined from stored blood samples which are usually hemolyzed. Of course, stored plasma or serum samples can be analyzed after separation from fresh blood samples for the BChE activity by the assay.
CONCLUSIONS
The assay is simple and faster than can be performed even on a semiautomatic colorimeter, so the present protocol is suitable for routine assays, particularly because it is suggested to measure both enzymes with the same substrate (ATCh), color reagent, and buffer solutions. It will be useful for clinical/pathological/toxicology laboratories for precise determination of AChE and BChE in blood in OP-intoxication where quicker results are required and analysis needs to be performed on the same day with fresh blood and also in laboratories related to toxicology. This simplified method for the determination of AChE and BChE in blood can be useful for diagnosis and management of OP-poisoned patients or in health surveillance of workers at risk of OP pesticide exposure particularly in Asian countries.