INTRODUCTION
Human exposures to lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As) have been reported to cause several adverse health effects under certain circumstances1-4. As reviewed by the Agency for Toxic Substances and Disease Registry (ATSDR), exposure to lead, particularly in children, was associated with several adverse effects, including neurological, renal, cardiovascular, hematological, immunological, reproductive, and developmental effects1. It was also noted that ‘no safe blood lead level in children has been identified’ and that, according to the U.S. Centers for Disease Control and Prevention (CDC), a lead blood level of 3.5 µg/dL was considered to be the reference level for elevated exposure1. Oral exposure to cadmium in humans was noted to damage kidneys and cause bones to become fragile2. For mercury, neurological and renal effects were consistently reported for all forms of mercury in epidemiological and animal studies, and several additional effects (e.g. cardiovascular, hematological, immunological, reproductive) were noted in animal studies3. Lastly, ingestion of arsenic in adults and children was reported to cause similar effects, including irritation of the gastrointestinal tract, decreased production of red and white blood cells, blood vessel damage, and dermatological effects (darkened skin and ‘corns’ or ‘warts’ on the palms, soles, and torso); however, in children, there was also some evidence of lower IQ scores due to chronic exposure to inorganic arsenic4. Additionally, according to the International Agency for Cancer Research (IARC), cadmium and arsenic are classified as Group 1 carcinogens (note: there is no evidence for cadmium carcinogenicity via oral exposure), lead is classified as Group 2A (inorganic) or Group 3 (organic) carcinogen, and mercury is classified as Group 3 carcinogen (note: methylmercury is classified as Group 2B carcinogen)5.
A recent news article by Perkins6, in 2025, reported on the testing of 50 toothpastes and 3 tooth powders conducted by Lead Safe Mama, a business focused on lead poisoning prevention6. According to the news article: 1) most products tested had detectable concentrations of lead; 2) some products had detectable concentrations of cadmium, mercury, and/or arsenic; and 3) only five products had non-detectable metal concentrations (shown in Supplementary file Table S1).
Toothpaste ingestion in children and adults has been documented in scientific literature. A systematic review and meta-analysis conducted by Petrović et al.7 revealed that the overall risk of systemic toxicity due to toothpaste ingestion was low and no severe or life-threatening events were reported in the studies reviewed by the authors; however, higher risk of dental fluorosis was associated with toothpaste formulations containing higher fluoride concentrations. Additionally, the authors documented several studies that evaluated toothpaste ingestion in children and adults. For example, toothpaste ingestion percentages in children were reported to be 27.6%8 and 35.5%9 of the amount applied. Moreover, in a randomized, single-blinded, crossover study conducted in three age groups (2–4, 5–7, and 8–12 years), higher toothpaste ingestion was noted in younger children, such that the average ingestion amounts were 0.205, 0.125, and 0.135 g in the three age groups, respectively10. In another study conducted in four age groups (2–4, 5–7, 11–13, and 20–35 years), toothpaste ingestion was reported to decrease with age, such that an average of 0.30, 0.13, 0.07, and 0.04 g/brushing was ingested by the four age groups, respectively11. Notably, the latter study was relied upon by the Dutch National Institute for Public Health and the Environment (RIVM) in its Cosmetics Fact Sheet when describing toothpaste use among various age groups12.
Given that heavy metals are reported in some toothpastes, and toothpaste ingestion is expected to occur in children and adults during tooth brushing, the current study conducted a risk assessment to evaluate whether health risks could be anticipated in children and adults due to the use of such toothpastes. The study focused on the testing data reported for the 50 toothpastes in the aforementioned news article. Tooth powders, which were also mentioned in the news article, were not considered in the current study, since they are less commonly used than toothpastes13.
COMMENTARY
Screening level risk assessment methodology
To better understand the potential public health significance of the data reported in the news article (i.e. ‘Does the presence of heavy metals in toothpastes represent a health risk to consumers?’), the reported results for 50 toothpastes noted in Supplementary file Table S1 were used to conduct a screening level risk assessment using the methods described in Supplementary file [note: the three tooth powders (i.e. products #50, 52, and 53) were not included in the assessment]. This screening level assessment focused on the most sensitive health endpoints for oral exposure for each of the metals of interest (non-cancer endpoints for lead, cadmium, and mercury, and cancer for arsenic). Briefly, the metal concentrations in ppb were divided by 1000 to obtain concentrations in µg/g. Subsequently, average daily doses (ADDs) were calculated for lead, cadmium, and mercury by considering the metal concentration in toothpaste, toothpaste ingestion rate, and number of brushings per day. For arsenic, the lifetime average daily doses (LADDs) were calculated by considering the metal concentration in toothpaste, toothpaste ingestion rate, number of brushings per day, body weight, as well as annual and lifetime exposure durations. Subsequently, the ADDs and LADDs were compared to the available health guidance values (HGVs) and hazard quotients (HQs) were calculated. For all metals, HQs greater than 1.0 were considered to represent an increased potential for health risk.
Screening level risk assessment results
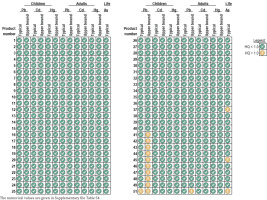
The screening level risk assessment showed that the ADDs for cadmium for all toothpastes under the typical and reasonable upper bound toothpaste use scenarios (see Supplementary file for additional details on the use scenarios) were lower than the respective HGVs, resulting in HQs <1.0 (Table 1 and Figure 1; and Supplementary file Table S4). For lead, the ADDs were greater than the HGV under reasonable upper bound use scenario in 10 toothpastes in children and one toothpaste in adults, as well as under typical use in one toothpaste in children. For arsenic, the LADDs were greater than the HGV for three toothpastes. Given the HGV exceedances for lead and arsenic, blood lead levels and dietary intakes of lead and arsenic were considered to better contextualize the exposure as discussed next.
Table 1
Exposure doses (µg/day) for lead, cadmium, and mercury in children and adults, as well as lifetime average daily doses (µg/kg/day) for arsenic, in 50 toothpastes. The doses were calculated using the heavy metal concentrations reported for the 50 toothpastes and applicable exposure assumptions
Figure 1
Summary of risk assessment findings for 50 toothpastes. The daily doses (μg/day) for lead, cadmium, and mercury in children and adults, as well as the lifetime average daily doses (μg/kg/day) for arsenic, are compared to the corresponding health guidance values. Doses below the health guidance values are expressed as green check marks, whereas doses exceeding the health guidance value are expressed with yellow exclamation signs
Blood lead levels and dietary lead intake
Although frequently identified as a health hazard, the consumption of lead in the human diet is unavoidable due to its occurrence in the Earth’s crust as galena and other minerals. The crustal occurrence and widespread commercial past and present uses of mined lead result in impurities in agricultural soil, feed, and crops, that cannot be feasibly reduced to non-detectable quantities1. Thus, regulatory agencies such as the U.S. Food and Drug Administration (FDA) have set action levels for adulteration in consideration of reference blood levels, but also ‘achievable by industry when control measures are taken to minimize the presence of lead’14. The FDA interim reference level (IRL) values for total dietary intake of lead of 2.2 and 8.8 μg/day for children and women of child-bearing age, respectively, were set based on the CDC blood lead reference value (BLRV) of 3.5 µg/dL derived by CDC and a 10-fold safety factor15. For example, FDA’s 10 ppb action level for fruits, vegetables, mixtures, yogurt, and meat set based on technical feasibility corresponds to modeled 90th percentile lead intake in babies of 0.61 µg/day, which, when considered with other food categories, should not result in an exceedance of the BLRV.
Using a similar approach as FDA has recently followed for setting lead action levels for baby foods, it is possible to evaluate whether the range of purported detected concentrations of lead in toothpaste could cause exceedances of BLRV. Considering the recommended toothpaste usage for a young child (aged 1–2 years) of pea-sized amount of toothpaste (i.e. 0.25 g) twice per day and a hypothetical action level of 1000 ppb lead, toothpaste would be unlikely to result in discernible increases in blood lead concentration (within tolerances of administered blood lead tests) or explain occurrences of blood lead poisoning in young children (Table 2). In the recently publicized testing, only one toothpaste with concentration exceeding 1000 ppb was identified. An action level of 1000 ppb for toothpaste would be consistent with the recently effective Toxic-Free Cosmetics Act (‘TFCA’) in Washington State, with a statutory limit of 1000 ppb. Although the Washington State Department of Ecology has issued interim guidance acknowledging that a 1000 ppb ‘limit can be difficult, if not impossible, to achieve in some products’16, such a limit would appear to be appropriate for toothpastes intended for use by children.
Table 2
Comparison of lead intake from toothpaste to the interim reference level and baby food action level scenarios. To contextualize the lead exposure in children due to toothpaste ingestion, the lead concentrations in toothpaste are expressed as incremental blood lead increases and compared to other sources of lead in children
| Scenario | Baby and young child FDA action level or toothpaste Pb concentration (ppb) | Intake (µg/day) | Incremental blood lead increase (µg/dL) | Percent BLRV |
|---|---|---|---|---|
| FDA IRL | ||||
| FDA Basis of 2.2 µg/day Interim Reference Level | Not applicable | 2.2 | 0.35 | 10 |
| FDA action level (90th percentile intake) | ||||
| Fruits, vegetables (excluding single-ingredient root vegetable products), mixtures, yogurts, custards/puddings, single-ingredient meat | 10 | 0.61 | 0.098 | 2.8 |
| Single-ingredient root vegetables | 20 | 0.62 | 0.099 | 2.8 |
| Dry infant cereals | 20 | 0.23 | 0.037 | 1.1 |
| Hypothetical toothpaste Pb concentrations or future action levels | ||||
| Toothpaste – pea sized amount (0.25 g) twice per day | 10 | 0.005 | 0.0008 | 0.0 |
| 100 | 0.05 | 0.008 | 0.2 | |
| 500 | 0.25 | 0.04 | 1.1 | |
| 1000a | 0.5 | 0.08 | 2.3 | |
| 5000 | 2.5 | 0.4 | 11 | |
| 10000b | 5 | 0.8 | 23 | |
| 20000 | 10 | 1.6 | 46 | |
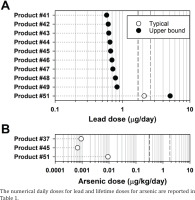
Additionally, lead ADDs for 10 toothpastes exceeding the HGV in children were compared to the mean and 90th percentile total dietary lead intakes of 1.7 and 2.6 µg/day, respectively, reported in children (1–3 years)15. With the exception of one toothpaste, the lead ADDs were markedly lower than the total dietary lead intakes (Figure 2A).
Figure 2
Comparison of lead and arsenic doses from toothpastes that had health guidance exceedance to dietary lead and arsenic intakes. To contextualize the lead and arsenic exposure due to toothpaste ingestion, the lead doses that exceeded the health guidance values in 10 toothpastes in children are compared to the 90th percentile lead dietary values of 1.7 and 2.5 μg/day, respectively (vertical dashed lines) (A), and the arsenic lifetime average daily doses that exceeded the health guidance values in three toothpastes are compared to the lowest and highest mean dietary arsenic values of 0.3 and 1.8 μg/day (vertical dashed lines) (B)
It is noteworthy that in addition to dietary exposure, lead exposure in children could also occur via ingestion of dust due to normal hand-to-mouth activity (baseline estimate of dust ingestion in children aged 1–6 years was 0.2 g dust per day) and/or soil due to pica (i.e. ingestion of inedible items) (children engaging in pica could ingest as much as 5 g soil per day)1. In a study by Zartarian et al.17, the median and 95th percentile dust lead concentrations were 72 and 320 µg/g, respectively, whereas the median and 95th percentile soil lead concentrations were 26 and 426 µg/g, respectively. This indicates that children could be exposed to 64 and 2130 µg/day lead from dust and soil, respectively (assuming the 95th percentile concentrations), indicating that, in comparison to lead exposure from dust and/or soil, toothpaste ingestion in children was unlikely to be a significant source of lead exposure.
As for adults, a lead ADD of 0.84 µg/day noted for one toothpaste was the only ADD to exceed the HGV. This ADD was 10.5-times lower than the aforementioned FDA IRL of 8.8 μg/day for women of child-bearing age. Further, assuming a body weight of 70 kg, this ADD equaled 0.012 µg/kg/day, which was 42-times lower than the average dietary lead intake of 0.5 μg/kg/day reported for European consumers aged ≥18 years by the European Food Safety Authority (EFSA)18. Notably, the HGV used for lead is intended to be protective of children and pregnant women and use of this HGV in risk assessment is likely to overpredict risks to adults.
Dietary arsenic intake
Arsenic is a naturally occurring element widely distributed in the Earth’s crust4. It is usually found in the environment combined with other elements such as oxygen, chlorine, and sulfur; arsenic combined with these elements is inorganic arsenic. Arsenic combined with carbon and hydrogen is organic arsenic. Inorganic arsenic occurs naturally in the soil and minerals; thus, it can enter the air, water, and land from wind-blown dust and can get into water from runoff and leaching. Volcanic eruptions are another source of arsenic. Arsenic can also enter the environment from anthropogenic activities, such as mining and smelting of ores, combustion of coal at power plants and incinerators, and discharging of industrial wastes. Since arsenic is found naturally in the environment, humans are exposed to arsenic via food, water, or air, and children can also be exposed to arsenic via soil ingestion. Food is usually the largest source of arsenic intake, with the mean daily dietary intake ranging from 0.31 to 1.80 µg/kg/day across various group ages4.
Given that HGV exceedances were noted for arsenic in three toothpastes, it was important to compare the LADDs (i.e. 9.2×10-4, 6.8×10-4, and 9.1×10-3 µg/kg/day) to the average daily dietary arsenic doses. The LADDs were several orders of magnitude lower than the average daily dietary arsenic intakes of 0.31–1.80 µg/kg/day (Figure 2B), indicating that, in comparison to dietary arsenic consumption, toothpaste ingestion was unlikely to be a significant source of arsenic exposure.
Strengths and limitations
It is noteworthy that the screening level risk assessment relies on conservative assumptions. Likely, the main conservatism is the toothpaste ingestion rate. According to CDC, children (>3 to 6 years) should use ‘no more than pea-sized amount’ of toothpaste (or 0.25 g), whereas children (<3 years) should use a ‘smear of a rice grain’19. A toothpaste amount of 0.25 g is 1.2- and 2.9-times lower than the mean (i.e. 0.30 g) and 90th percentile (i.e. 0.73 g), respectively, of ingested toothpaste in children used in the screening level risk assessment. Thus, the metal doses in children calculated in our assessment were overestimated relative to recommended usage, especially relative to the 90th percentile ingestion rate. In fact, if a toothpaste amount of 0.25 g was used instead of 0.30 g and all was ingested (rather than the expected fraction of the amount applied), there would be only one HGV exceedance for lead in children (product #51) and two HGV exceedances for arsenic (products # 37 and 51) (data not shown). This also indicates that ensuring the use of a ‘pea-sized’ amount of toothpaste could be an effective measure to minimize exposure to metals in toothpaste.
In addition to assumptions about the amount of toothpaste ingested, the HGVs used also represent conservative selections. For lead, the maximum allowable daily limit (MADL) of 0.5 µg/day derived by California’s Office of Environmental Health and Hazard Assessment (OEHHA) was used20. This HGV is specifically derived to be protective of developmental effects of lead and is most applicable to children and pregnant women, who may be most sensitive to the effects of lead on brain development. Notably, this HGV is 4.4-times lower than the aforementioned FDA IRL of 2.2 µg/day in children. When comparing ADDs to the FDA IRL, only one toothpaste evaluated in this assessment would represent a potential for increased health risk under the reasonable upper bound use. Similarly, for arsenic, we relied upon an oral cancer slope factor (CSF) developed by U.S. Environmental Protection Agency (EPA) Integrated Risk Information System (IRIS) for inorganic arsenic21. However, the testing conducted on arsenic in this study did not speciate inorganic and organic forms. Organic arsenic has a reduced toxicity profile and different dose-response to that of inorganic arsenic, such that the use of a benchmark specific to inorganic arsenic has the potential to overestimate risk when the nature of the arsenic present is unknown.
Another conservative assumption, especially for toothpastes with higher metal concentrations, is that children and adults use the same toothpaste over prolonged periods. The reported range of concentrations of lead and arsenic in toothpastes spanned several orders of magnitude. Although no statistics were found on how many brands of toothpaste a typical household uses over a period, it would be reasonable to expect that toothpaste selection/use could be influenced by factors like product availability (i.e. an individual using one brand of toothpaste may purchase a different brand if the preferred brand is not available) and price (i.e. an individual using one brand of toothpaste may purchase a different brand if it is on sale and is more affordable than the preferred brand). Therefore, it is possible that children and adults vary toothpaste brand, which would affect the exposure to metals that may be present in toothpaste, based on the high variability in concentration found across products.
Furthermore, some uncertainties and limitations also originate from the dataset used in the assessment. A key limitation is replication. Given that a single concentration was reported for each metal and for each product, it is unknown how many times the products were tested and/or if multiple lots or batches of the product were tested. If the products were tested multiple times (ideally at least three different lot numbers), these data would help to understand the consistency of the findings and increase the certainty of the risk implications. Generally, the minimal number of independent replicates required for statistical analysis is three (i.e. n=3), with a greater number of replicates often needed to detect statistically significant differences. It is possible that the metal concentrations in the same toothpaste could vary across product batches/lots. Consequently, the exposure to metals present in the same toothpaste could also vary. The other limitations associated with the dataset is the lack of information on the laboratory that conducted the testing (it is essential that the laboratory has proper accreditation), specific testing methods that were used (many analytes have standardized testing methods that are important to follow), as well as chain of custody (i.e. by whom and how the products were purchased, handled, shipped etc.).
CONCLUSION
A screening level risk assessment conducted using the testing results obtained by Safe Lead Mama showed that the cadmium and mercury ADDs for 50 toothpastes were markedly lower than the HGVs, and the HQs were less than 1.0. For lead, the ADDs exceeded the HGV in 10 toothpastes (children’s) and one toothpaste (adults’) under reasonable upper bound use scenario, and one toothpaste (children’s) under typical use scenario. Additional analyses showed that blood lead levels in children ingesting toothpaste would be low and that lead doses from toothpaste ingestion were lower than the dietary lead intakes in children and adults. For arsenic, the LADDs exceeded the HGV for three toothpastes; however, these LADDs were several orders of magnitude lower than the dietary arsenic intakes. It was concluded that the presence of the four heavy metals in the toothpastes tested is not anticipated to increase health risk.


