INTRODUCTION
Kratom (Mitragyna speciosa) is a tropical tree indigenous to Southeast Asia with a long history of traditional use in parts of Africa and Southeast Asia. The plant’s leaves have been historically used to manage pain, opioid withdrawal symptoms, and to combat fatigue1,2. Also known as thom, thang, ketum, and biak, kratom has gained significant popularity worldwide as both a stimulant and an opioid substitute, consumed in various forms including tea, chewed leaves, smoked material, or ingested capsules2,3. Despite its increasing prevalence, kratom remains largely unregulated in many countries, including the United States at the federal level, although some states have implemented bans4. As of 2025, kratom and its psychoactive component had been classified as Schedule I controlled substances in Alabama, Arkansas, Indiana, Rhode Island, Vermont, Wisconsin, and the District of Columbia5.
The pharmacological profile of kratom is complex and dose‐dependent. Its primary active compounds, mitragynine and 7-hydroxymitragynine, function as partial agonists at mu-, delta-, and kappa-opioid receptors in the central nervous system6. At lower doses, kratom produces stimulant effects, while higher doses lead to analgesic, euphoric, and sedative outcomes1,2,6. This dual action has contributed to its appeal as an alternative medicine for chronic pain management and as a self-treatment option for individuals experiencing opioid withdrawal symptoms7.
As kratom use has expanded in Western countries, there has been a corresponding increase in reports of adverse effects. The rate of calls to Wisconsin Poison Center during 1 January 2010 to 1 September 2022 regarding kratom exposures increased from 1 call in 2011 to a peak of 15 calls in 20208. Clinical manifestations of kratom toxicity include tachycardia, agitation, drowsiness, nausea, hypertension, and more severe complications such as seizures, psychosis, hepatotoxicity, and death1-3,6,9
Despite these concerning reports, the relationship between kratom use and seizure activity remains incompletely characterized. The clinical manifestations of kratom effects are not well‐defined, and formal studies are limited. Most of the current understanding comes from individual case reports, which vary in detail and clinical context.
This systematic review aims to comprehensively examine published case reports and case series of seizures associated with kratom use to better characterize this neurological complication. By analyzing the clinical presentations, dose considerations, concomitant substance use, and outcomes described in these reports, we seek to identify patterns that may inform clinical practice, public health interventions, and future research directions. Given the increasing popularity of kratom and the potentially life-threatening nature of seizures, a systematic evaluation of this association is both timely and necessary for healthcare providers, researchers, policymakers, and consumers.
METHODS
Literature search strategy
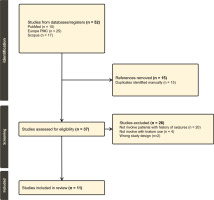
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 guidelines (Figure 1). A comprehensive literature search was performed by the author using PubMed, Europe PMC, and Scopus databases to identify all relevant case reports describing seizures associated with kratom use. The search included studies published in English up to 2025. Keywords and Medical Subject Headings (MeSH) used included ‘kratom’, ‘Mitragyna speciosa’, ‘seizure’, ‘epilepsy’, and ‘case report’. In PubMed, the specific query was: (‘Kratom’ OR ‘Mitragyna speciosa’) AND (‘seizure’ OR ‘epilepsy’ OR ‘convulsion’), with filters to exclude reviews. Similar Boolean strategies were applied to Europe PMC and Scopus with filters for human studies and case report‐type publications. The PRISMA checklist and the JBI critical appraisal checklist for case reports, are provided in the Supplementary file.
Study eligibility
Articles were included if they met the following criteria: 1) case reports or case series involving human subjects who experienced seizures temporally associated with kratom use; and 2) sufficient detail regarding the clinical presentation, kratom consumption pattern, and diagnostic evaluation. Exclusion criteria were: 1) animal or in vitro studies, 2) pharmacological or mechanistic studies without clinical data; and 3) narrative reviews, editorials, or articles lacking original patient data.
Data extraction and synthesis
The author reviewed all retrieved articles for eligibility. Screening of titles and abstracts was conducted using Rayyan10. Relevant data were extracted into a structured table that included publication details, patient demographics, kratom usage (dose, duration, route), co-ingestants, seizure characteristics, diagnostic evaluations (e.g. EEG, neuroimaging, toxicology), treatment modalities, and clinical outcomes. Data were synthesized using descriptive statistics (e.g. median, range, frequency counts) to summarize demographic and clinical variables. Additionally, qualitative pattern analysis was performed to identify recurring themes such as the type of seizures, common co-ingestants, and frequent management strategies including the role of kratom cessation.
RESULTS
A total of 52 studies were identified through systematic database searches (PubMed: 10, Europe PMC: 25, Scopus: 17). After the removal of 15 duplicates, 37 unique records remained for screening. All 37 studies were assessed based on title and abstract, followed by full-text evaluation. Of these, 26 studies were excluded for the following reasons: 20 did not involve patients with a history of seizures, 4 did not involve kratom use, and 2 were excluded due to unsuitable study design. This resulted in 11 studies that met the inclusion criteria (Figure 1). The quality of these included studies was assessed using the JBI Critical Appraisal Checklist for Case Reports11.
A total of 10 case reports12-21 and 1 case series22 (encompassing 21 individual patients) were included in this systematic review. Of these, 20 were adult cases and 1 neonatal21. Males comprised the majority in adult cases, with only 1 female case12. Further details about the included studies are presented in Table 1.
Table 1
Summary of published case reports and case series of kratom users with history of seizures
| Authors Year Country | Study design | Age (years) Sex | Kratom use | Seizure type | Co-substances/ Tox screen | EEG/ Imaging | Comorbidities | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Tassavor et al.12 2025 USA | Case report | 34 F | Paste, 6–8 tsp/day for 12 years | Not specified | Multiple IV drug use | Not reported | Untreated Hepatitis C | Levetiracetam | No new seizure |
| Holton13 2024 USA | Case report | 36 M | White vein kratom about 100 g/day for 3 years | Not specified | Alcohol, opioids | Not reported | Bipolar, Depression | Lamotrigine (non-adherent) | Not reported |
| Noe et al.14 2023 USA | Case report | 26 M | Kratom, polysubstance (acute) | GTC seizures, agitation | Benzodiazepines, LSD, MDMA, cannabis | CT: normal | Severe rhabdomyolysis, Acute kidney injury, Acute liver injury, Altered mental status/Agitation | Midazolam, dexmedetomidine, kratom cessation | Resolved, discharged stable |
| Patel et al.15 2021 USA | Case report | 28 M | 1 week escalating kratom use | Not specified | Alcohol, opioid use | MRI: cortical infarcts | Severe rhabdomyolysis, Acute renal failure, Severe metabolic acidosis, Acute liver injury, Cerebrovascular accident (multifocal infarcts), Transient nonischemic reversible cardiomyopathy, Pneumonia (MRSA), Impaired mental status | Supportive, kratom cessation | Recovered without further seizure |
| Afzal et al.16 2020 USA | Case report | 27 M | 3–4 kratom bottles/day for 1.5 years | GTC seizures | Opioid, diphenhydramine, benzodiazepine | CT/MRI: normal | Anxiety disorder, ADHD, Racing thoughts, insomnia | Supportive care, kratom cessation | Full recovery |
| Hughes et al.17 2019 USA | Case report | 27 M | Unknown amount | Not specified | Quetiapine | Not reported | Asperger syndrome, Bipolar disorder | N/A (found deceased) | Death |
| Tatum et al.18 2018 USA | Case report | 19 M | Daily kratom pills (chronic) | GTC and focal seizures | Marijuana, lisdexamfetamine dimesylate, alcohol, alprazolam | EEG: bilateral spikes; MRI: globus pallidus T1 hyperintensity | Anxiety, Admitted drug abuse and dependence | LEV → switched to lamotrigine, kratom cessation | Seizure-free post-kratom cessation |
| Nelsen et al.19 2010 USA | Case report | 64 M | Kratom tea + other botanicals | Not specified | Datura stramonium, amitriptyline, oxycodone, alcohol | CT: possible frontal lesion | Chronic pain, Depression | Intubation, supportive, kratom cessation | No recurrence |
| Boyer et al.20 2008 USA | Case report | 43 M | Tea 4 times/day for 3.5 years | GTC seizure | Modafinil, hydromorphone | MRI/CT: normal | Chronic pain from thoracic outlet syndrome, Opioid dependence | Buprenorphine/ naloxone, kratom cessation | Seizure-free after stopping kratom |
| Spungen et al.21 2024 USA | Case report | Neonate | Maternal kratom + kava use during pregnancy | GTC seizure within 24 h | Nuprenorphine, tobacco (maternal) | Neonatal EEG: normal; imaging not specified | Prenatal exposure | Lorazepam, phenobarbital | Full resolution |
| Halim et al.22 2021 Malaysia | Case series | 17–29 11 M | Various dose and frequency, some mixed with diphenhydramine syrup | 7 GTC, 4 focal progressing to GTC | 4 patients mixed with diphenhydramine syrup, 1 patient used methamphetamine (4 days prior), 2 patients positive for opioids, 1 patient positive for ATS | EEG: 5 abnormal CT: all normal | Drug abuse (intentional OD) | AEDs in 2 cases; rest supportive, kratom cessation | All recovered after abstinence |
The most common seizure type reported was generalized tonic-clonic (GTC). Focal seizures with secondary generalization were also described in some cases18,22.
In the case series of Halim et al.22, EEG was performed in 8 of 11 reports, with epileptiform activity observed in 5 patients, Tatum et al.18 case’s EEG bilateral spike was observed, while EEG of neonate in Spungen et al.21 case appeared normal. While others showed either normal findings or nonspecific slowing. Neuroimaging (CT/MRI) was reported in 10 studies, with most scans returning normal results or showing non‐epileptogenic findings. Only one case reported delayed imaging abnormalities18.
Kratom use duration ranged from a single week of escalation15 to over 12 years12, with chronic use (>1 year) reported in at least 9 cases. Doses were inconsistently reported but ranged from moderate (about 20 g/day) to extremely high (>100 g/day)13.
Polysubstance use or co-ingestion was reported in the majority of the included cases, with only a minority involving kratom use alone. Documented co-substances included opioids, benzodiazepines, alcohol, stimulants, and other prescription or illicit drugs. For example, co-ingestion of benzodiazepines, LSD, MDMA, and cannabis was reported in a patient with generalized tonic-clonic seizures14, while another case involved chronic kratom use with alcohol and opioid co-use13. Additional substances reported include diphenhydramine and quetiapine16,17, as well as modafinil and hydromorphone20. In a case series, multiple patients had co-ingestion of diphenhydramine, methamphetamine, or possible opioid exposure22. Only a limited number of cases reported kratom use without confirmed co‐substance involvement, though the presence of undetected substances cannot be excluded18,22.
Qualitative trends
Several qualitative trends were identified across the included case reports. Many cases18,20 reported chronic, escalating kratom consumption prior to the onset of seizures. Continued kratom use often resulted in breakthrough seizures, and cessation of kratom use was temporally associated with seizure resolution in multiple cases16,18,22. A key therapeutic intervention was cessation, as kratom discontinuation was explicitly linked to improved outcomes or seizure freedom in at least 8 studies15,16,20,22. In several of these, cessation occurred alongside supportive care or antiepileptic drug initiation. Confounding by polysubstance use was also noted as many patients presented with a history of or concurrent use of other substances, particularly opioids and benzodiazepines13,14, complicating direct attribution of seizure activity to kratom alone.
In general, the included studies had a lack of dose standardization, as the variability in the forms of kratom (e.g. powder, tea, paste, capsules) and lack of quantifiable dosing in many reports limited comparative conclusions regarding dose thresholds for seizure risk.
Finally, one notable case21 highlighted in utero exposure, with the neonate experiencing seizures shortly after birth, suggesting possible kratom-related neurotoxicity or withdrawal in perinatal contexts.
DISCUSSION
This systematic review summarizes published case reports that link kratom use to seizure activity, drawing from 11 reports and 21 total patients. Our findings suggest that chronic, high-dose kratom use may be associated with an increased risk of seizures, particularly generalized tonic-clonic seizures, and that cessation of kratom appears to be a key component of effective management.
Principal findings
The most frequently reported seizure type was generalized tonic-clonic, with fewer cases describing focal seizures or ambiguous seizure-like activity. Many patients had a chronic pattern of use, often escalating over months to years, with several cases documenting daily consumption of high doses exceeding 20 g/day, and in one instance, exceeding 100 g/day13. The latency between initiation and seizure onset varied, but multiple reports noted seizures after prolonged kratom consumption, raising concern for either dose-dependent neurotoxicity or withdrawal-related effects18,20.
Importantly, in at least 8 of the 11 reports, cessation of kratom was either directly associated with cessation of seizures or recommended as a treatment alongside supportive care or antiepileptic drugs16,18,20,22. This supports the hypothesis that kratom-related seizures may be reversible upon drug discontinuation, although the exact mechanism remains unclear. Whether these seizures are provoked (toxic), unmasking an underlying predisposition, or withdrawal-mediated remains a matter of clinical interpretation.
Potential mechanisms underlying kratom-associated seizures
The pathophysiological mechanisms linking kratom use to seizures remain incompletely understood, but emerging evidence points to multifactorial interactions involving receptor pharmacology, polypharmacy, product variability, and individual susceptibility. Below, we compile findings from preclinical and clinical studies to propose plausible pathways.
Receptor-mediated neuropharmacological effects
Kratom’s primary alkaloid, mitragynine, exhibits a complex polypharmacological profile. While its partial agonism at μ‐opioid receptors (MOR) is well‐documented23,24, recent studies highlight its affinity for adrenergic‐α2 (Aα2R) and serotonin (5-HT) receptors, which may play a critical role in modulating seizure thresholds24,25. Mitragynine binds Aα2R with moderate affinity (Ki=32–39 nM), and adrenergic signaling is known to influence neuronal excitability. For instance, α2‐adrenoceptor agonists like clonidine can suppress seizures by hyperpolarizing noradrenergic neurons, but paradoxical excitatory effects may occur at higher doses or in specific circuits24. Similarly, mitragynine and its analog paynantheine demonstrate submicromolar affinity for 5‐HT1A and 5‐HT2B receptors. Dysregulation of 5-HT1A receptors has been implicated in both pro- and anti-convulsant effects depending on brain region and receptor density25. In rodent models, 5‐HT1A agonists reduce seizure severity in some paradigms but exacerbate activity in others, suggesting a bidirectional relationship that could explain variability in human case reports25,26.
Notably, 7-hydroxymitragynine, a metabolite of mitragynine with higher MOR efficacy, does not bind adrenergic or serotonergic receptors24. This divergence implies that raw kratom preparations – which contain both alkaloids –may exert competing effects: MOR activation (sedative, antinociceptive) versus Aα2R/5‐HT modulation (potentially proconvulsant)23,24. Such receptor-level interactions could lower seizure thresholds, particularly in individuals with genetic or acquired vulnerabilities in these pathways.
Polypharmacy and pharmacokinetic interactions
Co-ingestion of kratom with other substances was reported in 15 cases (71% of seizure cases in this review). Synergistic interactions with CNS depressants (e.g. benzodiazepines, opioids) or stimulants (e.g. modafinil, amphetamines) may destabilize neuronal networks. For example, a case of tonic-clonic seizures occurred after combining kratom with modafinil, a dopamine reuptake inhibitor20. Modafinil lowers seizure thresholds via glutamate release and histaminergic activation, while kratom’s serotonergic activity could further disrupt excitatory-inhibitory balance20,25. Similarly, concomitant opioid use can lead to serious central nervous system complications. Both substances activate μ‐opioid receptors, which may result in additive effects that amplify respiratory depression by reducing the medullary response to hypercapnia and decreasing respiratory rate and tidal volume27. Additionally, kratom’s α2‐adrenergic receptor agonism may interact with the sympathetic suppression induced by opioids27, leading to autonomic instability and an increased risk of hypoxia. This can initiate a cascade where respiratory depression leads to hypercapnia and hypoxemia, triggering cerebral vasodilation, increased intracranial pressure, and ultimately cortical irritation that may result in seizures23,27,28.
Withdrawal and neuroadaptation
Abrupt cessation of chronic kratom use can precipitate withdrawal syndromes characterized by hyperalgesia, agitation, and autonomic instability23. While kratom withdrawal is generally milder than classical opioid withdrawal, adrenergic hyperactivity during withdrawal may lower seizure thresholds. Rodent studies show that chronic MOR activation downregulates Aα2R expression, creating a rebound hyperadrenergic state upon discontinuation24. This mechanism parallels alcohol withdrawal seizures, where noradrenergic surges contribute to neuronal hyperexcitability24. Notably, several case reports of kratom-related seizures have documented concurrent alcohol use, as presented in reports13,15,18,19, which may act synergistically to lower the seizure threshold during kratom withdrawal or intoxication. Therefore, while alcohol was not always systematically reported, its presence in multiple cases highlights the need to consider additive risk from co-intoxicants when evaluating kratom-associated seizures.
Individual susceptibility
Case reports involved individuals with predisposing factors as mental disorders, as presented in reports13,16-19, associated with dysregulated HPA axis, lead to disrupt GABAergic signaling and promote neuroinflammation which potentiates seizure29-31.
Genetic variations in COMT (catechol-O-methyltransferase) and SLC6A4 (serotonin transporter), which are implicated in substance use disorders and neurotransmitter regulation, may influence individual susceptibility to seizures resulting from kratom use. Although direct studies on kratom‐specific genetic interactions are lacking, existing knowledge about these genes provides mechanistic insights.
The COMT Val158Met (rs4680) polymorphism alters dopamine metabolism. Individuals with the Met allele have reduced COMT enzyme activity, which leads to increased dopamine levels in the prefrontal cortex32,33. This heightened dopaminergic tone may enhance excitatory neurotransmission and lower the seizure threshold, particularly when exposed to psychoactive substances such as kratom that affect monoaminergic systems.
The SLC6A4 gene encodes the serotonin transporter (SERT)34, which is responsible for the reuptake of serotonin from the synaptic cleft into presynaptic neurons. Variants like the 5‐HTTLPR short (S) allele result in reduced transporter expression and increased synaptic serotonin35. Since kratom also influences serotonergic pathways, individuals with this genotype may experience exaggerated serotonergic responses, which could contribute to seizure risk.
These genetic factors may alter the pharmacodynamic response to kratom, potentially increasing the likelihood of adverse neurological effects such as seizures in genetically predisposed individuals.
Mitochondrial dysfunction and neuroinflammation
Emerging preclinical data suggest that mitragynine may impair mitochondrial biogenesis. In rat models, prolonged kratom exposure reduces peroxisome proliferator-activated receptor γ coactivator 1α (PGC‐1α), a key regulator of mitochondrial energy metabolism36. Mitochondrial dysfunction elevates reactive oxygen species (ROS), which activate NLRP3 inflammasomes‐a pathway implicated in pentylenetetrazole-induced seizures26,36.
Neonatal and unique presentations
The inclusion of a neonatal seizure case21 introduces concern for in utero exposure and neonatal withdrawal or toxicity, especially since the mother consumed high doses of kratom blends throughout pregnancy. While confounding factors (e.g. other substances, maternal comorbidities) exist, the temporal association and absence of other clear etiologies point to a possible kratom‐related mechanism. While direct evidence in humans is lacking, this mechanism could explain delayed-onset seizures in chronic users.
Clinical implications
Clinicians should consider kratom use in the differential diagnosis of new-onset seizures, particularly in patients with polysubstance use, psychiatric illness, or poorly explained clinical presentations. Given the over-the-counter availability of kratom and its perception as a ‘natural’ alternative to opioids or anxiolytics, it may be underreported or overlooked during clinical encounters. A thorough history, including inquiry about herbal and recreational substances, is essential.
When kratom‐related seizures are suspected, supportive care, toxicology testing, and patient education regarding cessation are crucial. EEG and neuroimaging can help rule out structural or epileptogenic causes, but in many reviewed cases, findings were nonspecific or normal.
Limitations
This review is limited by the nature of case reports, which are inherently subject to reporting bias, incomplete data, and variable diagnostic certainty. The inability to verify mitragynine levels in most cases, combined with inconsistent EEG/imaging documentation and the high prevalence of polysubstance use, makes definitive causality difficult to establish. Additionally, the small sample size and heterogeneity across cases preclude statistical analysis beyond descriptive summaries. It is also important to note that no formal risk of bias assessment for the individual case studies was performed.
Implications
Further research is needed to clarify the dose-dependent risk of seizures, identify vulnerable subpopulations, and determine the pharmacological basis for kratom-induced neurotoxicity. Prospective case series, registry studies, or pharmacovigilance analyses could help improve understanding of this growing public health issue. Additionally, awareness campaigns targeting healthcare providers and the public may reduce the risks associated with unregulated kratom use.
CONCLUSIONS
While evidence from case studies is limited and often confounded, this review suggests kratom use may be associated with seizure activity, especially in vulnerable populations or those with polysubstance use. Clinicians should inquire about kratom during seizure evaluations using detailed substance use history and, where feasible, laboratory screening. Although routine drug panels typically do not detect mitragynine, specialized liquid chromatography‐mass spectrometry (LC‐MS/MS) assays can be used to confirm kratom exposure. Further research, including controlled studies and standardized toxicology testing, is urgently needed to clarify risk profiles.