INTRODUCTION
According to the World Health Organization, the tobacco pandemic kills approximately 8 million people every year worldwide; it is one of the worst risks to public health that the world has ever faced1. Of those deaths, almost 7 million are directly related to tobacco use, and >1.3 million deaths are caused by passive smoke exposure to non-smokers1. Nicotine, reactive oxygen and nitrogen species (RONS), and several other components have been found in cigarette smoke causing significant health problems such as respiratory diseases and lung cancer. It also affects the vascular and other organs imposing severe risk for permanent health damage and death2. CS contains nicotine and other constituents which produce a high amount of reactive oxygen species (ROS) and causes an imbalance in the level of antioxidants in the body, thereby giving rise to oxidative stress3.
Cigarette smoke is a well-established risk factor in the development of chronic obstructive pulmonary disease (COPD), which is characterized by chronic airflow limitation associated with various extra-pulmonary manifestations, such as cardiovascular, metabolic, diabetic, muscular and bone disorders4. About 4000 distinct kinds of extremely complex and harmful agents, such as oxidants and other free radicals, are found in cigarette smoke5. Two key pathophysiological variables linked to the etiology of COPD are elevated oxidative stress and inflammation, which appear to be correlated with an increased risk of COPD development in smokers6.
In biological systems, RONS can either cause or facilitate oxidative damage7. The harmful effects of CS either through direct or passive inhalation induce highly toxic effects on health. The disturbance in the balance of oxidative stress levels leads to the initiation of serious health issues such as inflammation, cell death and senescence, and lung diseases, many of which need immediate attention8. Free radicals can often produce various oxidized bases and inflict oxidative damage to biological DNA. One of these significant oxidative DNA lesions, 8-oxo-2′-deoxyguanosine (8-oxodG), is created when 2′-deoxyguanosine’s C-8 position gets oxidize. It is frequently employed as a biomarker of oxidative DNA damage9. Production of 8-oxodG can result in DNA misreplication, which can cause mutations and cancer. Studies have shown that leukocytes in smokers, lung tissue, and urine contain greater amounts of 8-oxodG than those of non-smokers9.
It has been reported that acute cigarette smoking causes platelet reactions, such as the release of nucleotides, and markedly raises the epinephrine levels in the plasma7. By influencing platelets in several ways, extracellular adenine nucleotides like adenosine triphosphate (ATP), adenosine diphosphate (ADP), and nucleoside adenosine are reported to control the vascular response to endothelial injury. Adenosine is a strong inhibitor of platelet aggregation and a significant modulator of vascular tone, while the nucleotide ADP is the primary promoter of platelet aggregation7.
The ability of an organism to counter the effects of oxidative stress largely depends on the age group it belongs to. It has been observed that the anti-oxidant capabilities of an organism generally decline with advancing age due to the decline in the efficiency of the body’s anti-oxidant system10. Moreover, the repercussions of oxidative stress may be dealt differently by different age groups of organisms11. In the present study, we have performed a comparative evaluation of passive cigarette smoke among the three different age groups of rats (young, middle-aged, and old). This study focuses on establishing a possible relationship, if any, between the age of the organism and the extent of harmful effects caused by passive cigarette smoking.
METHODS
Experimental animals
Male Wistar rats of three different age groups, i.e. young (4 months), middle-aged (12 months), and old (24 months), were purchased from CSIR-Indian Institute of Toxicology Research, Lucknow, India. Rats were housed in a controlled environment at 22 ± 2 ℃ and relative humidity of 55 ± 15% with a 12-h light/12-h dark cycle, and they had free access to drinking water and nutrient-rich pellets. All animal care and experimental procedures were approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Institutional Animal Ethics Committee (IAEC/AU/2019(1)/09), University of Allahabad, India and follow the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The ARRIVE guidelines checklist for reporting this animal research was used and is provided in the Supplementary file.
Only male rats were used because in female rats, data might be very unpredictable due to hormonal variations associated with the female reproductive cycle12. After one week of acclimatization, 36 male Wistar rats were divided into six groups (n=6) as follows:
Group 1: Young male rats (aged 4 months, body weight = 115 ± 10 g)
Group 2: Young male control rats (aged 4 months, body weight = 115 ± 10 g)
Group 3: Middle-aged male rats (aged 12 months, body weight = 180 ± 10 g)
Group 4: Middle-aged male control rats (aged 12 months, body weight = 180 ± 10 g)
Group 5: Old male rats (aged 24 months, body weight = 220 ± 10 g)
Group 6: Old male control rats (aged 24 months, body weight = 220 ± 10 g)
Groups 1, 3, and 5 were exposed to passive cigarette smoke for 15 minutes once every day for 30 days and had free access to food and water. Groups 2, 4, and 6 were the respective controls and were not exposed to cigarette smoke at all. The control groups had free access to food and water.
Cigarette smoke generation
An experimental setup consisting of an exposure chamber was constructed to generate cigarette smoke. The exposure chamber was constructed using a plastic material of dimensions 56.4 × 38.5 × 37.1 cm. It had small openings of 371 × 40 mm in both extremities for ventilation. The rats were placed inside the exposure chamber and two cigarettes fixed in a metal holder were lit. A stopwatch was turned on, and the cigarettes were allowed to burn down fully within 15 minutes. The smoke generated inside the chamber was suctioned by a noiseless extractor fan to keep an airflow inside the chamber. A metal grille was placed on top of the cigarette holder to avoid direct contact with the cigarettes and, thus, to prevent the rats from injuring themselves. The inhalation exposure of our study was for different age groups of rats, used as a simulation of environmental tobacco smoke as experienced by non-smokers7.
Assessment of biochemical parameters
Blood collection, isolation of erythrocytes, plasma and serum
After 30 days of treatment, the rats were euthanized by standard protocol, and blood samples were collected by cardiac puncture into heparinized syringes.
Anesthetic procedure
A combination of ketamine and xylazine was used to induce general anesthesia. The anesthetic solution was freshly prepared by mixing ketamine (100 mg/mL) and xylazine (20 mg/mL) in a 10:1 ratio (v/v). The anesthetic was administered intraperitoneally (IP) at a dose of 80–100 mg/kg ketamine and 8–10 mg/kg xylazine, based on individual body weight13. Injections were performed using a 25-gauge needle in the lower right quadrant of the abdomen to avoid damage to visceral organs.
Following the injection, the animals were monitored continuously. Loss of the righting reflex and absence of response to toe-pinch were used to confirm induction of a surgical plane of anesthesia. Anesthetic depth was rechecked immediately before blood collection14.
Blood collection via cardiac puncture
Cardiac puncture is a terminal procedure that allows for the collection of large, high-quality blood volumes directly from the heart under deep anesthesia, making it ideal for biochemical and hematological analysis at the endpoint of experiments15. In contrast, intravenous (IV) blood sampling techniques, such as collection from the tail, jugular, or saphenous veins, are non-terminal and suited for serial sampling in longitudinal studies16. While cardiac puncture offers better sample purity and volume, IV methods are less invasive and allow for repeated measurements, though they require careful handling to minimize animal stress and ensure welfare17. Our study required large volumes of blood for various biochemical assays, as mentioned in the following sections. Therefore, cardiac puncture was preferred over intravenous blood sampling.
Cardiac puncture was performed as a terminal procedure under deep anesthesia. The rat was placed in dorsal recumbency, and the xiphoid process was used as a landmark. A 21–23 gauge, 1-inch needle attached to a 5 mL syringe was inserted at a 30–45° angle just left of the sternum into the thoracic cavity. The needle was directed toward the heart while negative pressure was applied until blood was aspirated. A maximum volume of 6–10 mL of blood was collected, depending on the animal’s weight16. Blood was transferred to heparin or serum-separator tubes as required.
The red blood cells and plasma were separated by centrifugation of the collected blood at 800g at 4℃ for 10 min, and subsequent removal of the upper buffy coat and 15% of packed red blood cells (PRBCs). Post-separation, the RBCs were washed thrice with ice-cold phosphate buffer saline (PBS) (10 mM; pH 7.4) and used for the assessment of oxidative stress parameters such as reduced glutathione (GSH), malondialdehyde (MDA), and plasma membrane redox system (PMRS). Plasma was frozen and stored at -80°C for analysis of biochemical parameters such as the ferric-reducing ability of plasma (FRAP), advanced oxidation protein products (AOPPs), and protein carbonyls (PCOs).
Following collection, euthanasia was confirmed via cervical dislocation and observation of the absence of respiratory movement and cardiac activity for at least 5 minutes18.
Lipid peroxidation assay in erythrocytes
By quantifying the amount of malondialdehyde (MDA) in erythrocytes, lipid peroxidation products were identified using the technique of Esterbauer and Cheeseman19. Three mL of PBS with 0.5 mM glucose (pH 7.4) was used to suspend 0.2 mL of PRBCs. One mL of 10% trichloroacetic acid (TCA) and two mL of 0.67% thiobarbituric acid (TBA) were also added to the 0.2 mL of suspended PRBCs, which were then heated for 20 min at 90–100℃ before being cooled. After five minutes of centrifuging the mixture at 1000g, the absorbance of the supernatant was measured at 532 nm. The extinction coefficient (e ¼ 153000 M-1 cm-1) was used to get the MDA concentration (in nmol/mL PRBCs).
Estimation of plasma protein carbonyl content
The Levine20 method was used to assess the carbonyl content of plasma proteins. Two tubes containing plasma samples from each group were designated as a test and a blank control. The test sample received 4.0 mL of a reagent cocktail consisting of 10 mM 2,4-dinitrophenylhydrazine (DNPH) produced in 2 M HCl, while the blank control received 4.0 mL of 2 M HCl alone. After vortexing, the contents were incubated at 37℃ for one hour in the dark. To promote protein reactions, the tubes were shaken sporadically every 10 min. After adding 20% TCA (w/v) to each tube, the mixture was allowed to sit in ice for 10 min.
The protein pellets that accumulated at the tube’s bottom were then separated by centrifuging the tubes for 20 min at 850g. The supernatant was disposed of with care. Protein pellets were extensively cleaned with an ethanol-ethyl acetate (1:1, v/v) solution to eliminate any remaining DNPH and lipid remains. The protein pellets were then incubated for 10 min at 37℃ after being dissolved in 6 M guanidine hydrochloride. By measuring the supernatant’s spectra at 370 nm and comparing each sample to the control, the carbonyl content was calculated. The Lowry et al.21 technique was used to calculate the plasma total protein content. An absorption coefficient of 22000 M-1 cm-1 was used to determine the carbonyl concentration, and the results were reported in nmol/mg protein.
Estimation of AOPPs (advanced oxidation protein products) in plasma
With a small modification, the Witko-Sarsat et al.22 method was used to measure the amount of AOPPs in plasma. After diluting the initial plasma five times, 10 mL of 1.16 M potassium iodide were added to 2 mL of plasma. Twenty mL of glacial acetic acid were then added to the solution after two minutes. At 340 nm, the reaction mixture’s absorbance was measured instantaneously. A volume of 2.0 mL of chloramine-T solution (0–100 mmol/L) was used as a calibrator, while 2 mL of PBS served as a blank. Mmol of chloramine-T equivalents per liter of plasma (mmol/L) was used to express the amount of AOPPs present.
Determination of erythrocytes plasma membrane redox system (PMRS) activity
As previously reported by Rizvi et al.11, the reduction of ferricyanide was used to evaluate the PMRS activity in erythrocytes. To reach a final volume of 2 mL, PRBCs (0.2 mL) were suspended in PBS that contained 5 mM glucose and 1 mM newly made potassium ferricyanide. After 30 min of incubation at 37℃, the suspensions were centrifuged for 10 minutes at 800g. Using 4, 7-diphenyl-1, 10-phenanthroline disulfonic acid disodium salt, the ferro cyanide content of the collected supernatant was determined. The absorbance was measured at 535 nm (e ¼ 20500 M-1 cm-1). The results are reported in µmol ferrocyanide/mL PRBC/30 min.
Evaluation Of FRAP (total antioxidant capacity of plasma)
With minor adjustments, the Benzie and Strain23 method was used to assess plasma’s capacity to reduce ferric acid; 300 mM acetate buffer (pH 3.6), 20 mM ferric chloride, and 10 mM 2,4,6-tripyridyl-s-triazine (made in 40 mM hydrochloric acid) were combined in a 10:1:1 ratio to create the FRAP reagent. A volume of 3 mL of FRAP reagent were combined with 100 μL of plasma, and the mixture was thoroughly mixed. Using a UV-VIS spectrophotometer (Evolution 201, Thermo Fisher), the absorbance was measured at 593 nm every 30 s for 4 min. Using the standard calibration curve, the plasma’s FRAP values (mmol Fe (II) per L) were determined.
Evaluation of reduced glutathione and total plasma thiol
The procedure outlined by Garg et al.24 was used to measure the amount of reduced glutathione in erythrocytes, and the published method25 was used to estimate the plasma-SH groups. Based on the -SH group’s capacity to reduce 5, 50-dithiobis, 2-nitrobenzoic acid (DTNB) and produce a yellow-colored anionic product, the optical density of which was measured at 412 nm, both evaluations were made. The standard plot was used to determine the GSH concentration, which is given as mg/mL PRBCs. The unit of measurement for the -SH group concentration is nmol/mL of plasma.
Sialic acid (SA) level
The Spyridaki and Siskos26 approach was used to calculate the SA content of the erythrocyte membrane. The amount of membrane SA is reported as mg/mg membrane protein, and the quantity of sialic acid was determined by creating a calibration graph using standard solutions of SA.
Protein carbonyl (PCO) level
Erythrocyte membrane PCO was measured according to the procedure of Levine et al.20. Absorbance was measured at 370 nm and the amount of PCO was calculated using an extinction coefficient of 22000 M-1 cm-1. The concentration of PCO is expressed as mmol/mg membrane protein.
Statistical analysis
The statistical analysis was performed using Graph-Pad PRISM version 5.01 software. The values reported are expressed as mean ± standard deviation (SD). The statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test. A p<0.05 was considered statistically significant.
RESULTS
Effect of cigarette smoke generation on the antioxidant levels in young, middle-aged and old rats
Effect of cigarette smoke on FRAP level
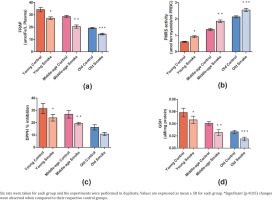
With respect to control rats, a significant decrease (p<0.05) is observed in the FRAP level in the groups inhaling smoke (YC=662.18, YS=527.53, MC=553.84, MS=396.10, OC=372.74, OS=277.59) (Figure 1). This decrease can be attributed to the increase in oxidative stress and, therefore, a gradual decline in the endogenous antioxidant levels.
Fig 1
Effect of cigarette smoke on the antioxidant parameters in RBCs and plasma of rats: a) The ferric reducing ability of plasma (FRAP) value gives the antioxidant capacity measured in the plasma of rats (μmol Fe/L plasma); b) The plasma membrane redox system (PMRS) measures the overall redox balance in erythrocytes of rats exposed to cigarette smoke (μmol ferrocyanide/mL PRBC); c) The effect of cigarette smoke on young, middle-aged, and old rats in terms of DPPH % inhibition; d) The intracellular GSH level (mg/mL) in RBC
Effect of cigarette smoke on PMRS activity
Our observed results show a significant increase (p<0.05) in PMRS levels in the treated rats in comparison to the control group (YC=0.989159892, YS=1.513279133, MC=2.210840108, MS=3.035772358, OC=3.462872629, OS=4.141463415) (Figure 1). This suggests an activation of PMRS in response to a disturbed redox state caused due to cigarette smoke.
Effect of cigarette smoke on plasma DPPH radical scavenging activity
The percentage inhibition in terms of DPPH radical scavenging was found to be significantly decreased (p<0.05) in each of the three groups, i.e. the young group, middle-age group, and old-age group when compared to their respective control groups (YC=31.57%, YS=24.04%, MC=26.81%, MS=19.33%, OC=16.32%, OS=10.84%) (Figure 1). However, on comparing the three groups, the percentage inhibition was recorded highest in the young group (24.04%), and intermediate in the middle-age group (19.33%). Interestingly, it was lowest in the old-age group (10.84%) suggesting an age-dependent loss of antioxidant activity.
Effect of cigarette smoke on GSH level
GSH is a strong determinant of the redox state and the intracellular GSH level is significantly reduced (p<0.05) in the smoke-treated groups amongst all age groups compared to their respective control groups (YC=0.0217875, YS=0.0173055, MC=0.015189, MS=0.009462, OC=0.00996, OS=0.0056025) (Figure 1). The decrease in GSH levels denotes a reduction in antioxidant levels and increased oxidative stress.
Effect of cigarette smoke on plasma protein oxidation level
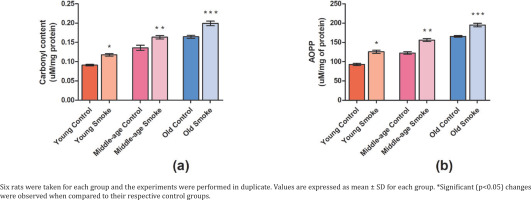
Protein carbonyl (PCO) content is significantly increased (p<0.05) in all the smoke-treated groups of different age groups when compared to their respective controls (Figure 2). Advanced oxidation protein products (AOPP) show levels of protein oxidation products which have significantly increased (p<0.05) in the treated groups compared to control rats (YC=26731.89, YS=36235.97, MC=35353.47, MS=44951.55, OC=47646.04, OS=56132.37) (Figure 2). The increase in oxidized protein products amongst all groups suggests an equally negative impact of cigarette smoke on health due to a rise in oxidative stress.
Fig 2
Effect of cigarette smoke on the anti-oxidant parameters in RBCs and plasma of rats: a) Protein carbonyl content in the plasma of control rats and cigarette smoke-treated rats (mmol/mg protein); b) Advanced oxidation protein products (AOPP) level in plasma of young, middle-aged, and old rats treated with cigarette smoke (μmol/L plasma)
Effect of cigarette smoke on plasma paraoxonase-1 aryl esterase (PON-1)
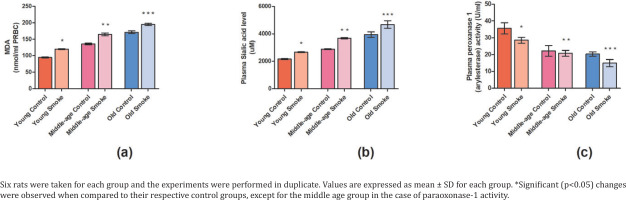
Our results showed a significant decrease (p<0.05) in the plasma paraoxonase-1 activity in all the cigarette smoke-treated groups in comparison to their respective control groups (YC=0.097484964, YS=0.078130126, MC=0.060843813, MS=0.056843448, OC=0.05562238, OS=0.04080554) (Figure 3). Further, the old age treated group showed the lowest PON-1 activity when compared to young age and middle age groups.
Fig 3
Effect of cigarette smoke on the anti-oxidant parameters in RBCs and plasma of rats: a) Lipid peroxidation content (nmol/mL RBC) is measured in terms of MDA levels. The effect of cigarette smoke on young, middle-aged, and old rats in terms of: b) sialic acid content (mM) in plasma; c) plasma paraoxonase-1 activity (U/mL)
Effect of cigarette smoke on plasma sialic acid content
Sialic acid content was found to be significantly increased (p<0.05) in all the cigarette smoke-treated groups of different age groups when compared to their respective controls (YC=1246242.77, YS=1538535.64, MC=1663391.13, MS=2126011.56, OC=2282466.28, OS=2700192.67) (Figure 3). However, the highest sialic acid content was found in the old age group and the lowest in the young age group, while the middle age group showed intermediate sialic acid content.
Effect of cigarette smoke on malondialdehyde content in erythrocytes
MDA is a content of lipid peroxidation and our results show significant increase (p<0.05) in MDA content in the treated rats with respect to control group (YC=9679.95, YS=12317.79, MC=13946.10, MS=16913.51, OC=17606.13, OS=19989.28) (Figure 3). The result suggests an increase in malondialdehyde content due to increased oxidative stress.
DISCUSSION
Cigarette smoke causes an increase in oxidative stress levels and disturbs the redox balance in the blood and plasma of organisms. A vast majority of antioxidant defense also relies on vitamin C which is particularly reduced due to its depletion and no further recycling during aging. The increase in PMRS during aging is a defense mechanism to recycle the extracellular ascorbate and reduce free radicals11. During conditions of oxidative stress caused by cigarette smoke, the obtained results show PMRS is significantly upregulated amongst all age groups. This denotes a similar condition as during aging-induced oxidative stress and the ability to recycle ascorbic acid during cigarette smoke-induced oxidative stress causes an upregulation in the PMRS levels in rats27.
The FRAP assay quantifies the reducing capacity of an antioxidant. It is a reliable way to measure the anti-radical activity of plasma. Higher FRAP levels indicate higher antioxidant capacity of the system. In our study, the higher FRAP levels of the cigarette smoke-treated groups when compared to their respective controls clearly explain that the anti-oxidant capabilities of the exposed groups have been compromised due to the passive smoke inhalation. Another correlation can be deduced from the present data, i.e. the FRAP assay values were found to be highest in YS and lowest in OS while the MS showed the intermediate value compared to YS and OS. The possible reason for the observed trend may be due to the age factor, which suggests that antioxidant capacity decreases with advancing age. These data suggest that the antioxidant capabilities were highest in YS, intermediate in MS, and lowest in OS.
The DPPH assay’s foundation lies in determining how well antioxidants can scavenge it28. Our study shows that the percentage inhibition of DPPH radicals was lowered in the cigarette smoke-exposed groups, i.e. YS, MS, and OS when compared to their respective control groups. These data suggest that the antioxidant capabilities of the exposed groups have deteriorated due to passive cigarette smoke inhalation. The effect can be seen in each of the exposed groups in terms of DPPH percentage inhibition. However, the data also show that the impact of passive smoking is also age-dependent. This can be understood by the fact that DPPH percentage inhibition was found to be lowest in the OS, intermediate in the MS, and highest in the YS group. The lower percentage inhibition of DPPH radicals in the smoke-exposed old age group compared to the young age group may be explained by the possibility that the antioxidant ability may decrease with advancing age.
GSH protects cells from oxidative damage and maintains redox equilibrium. During oxidative stress, the GSH levels are lowered in rats, altering the redox equilibrium29. The present study found that the young smoke (YS) group showed the highest value of GSH, the old smoke (OS) group showed the lowest value whereas the middle-aged smoke (MS) group showed the intermediate value of GSH. These data suggest that the effect of smoking was most prominent in the old smoke group when compared to the other two groups.
PON-1, often referred to as arylesterase (ARE) in human serum, is a vital antioxidant defense mechanism against lipid oxidation. As a measure of lipid peroxidation, PON-1 is a calcium-dependent esterase that is both anti-inflammatory and antioxidant and is linked to high-density lipoprotein (HDL)30. It has been found that the decrease in PON-1 activity is linked to elevated oxidative stress, thus it is used as a marker of oxidative stress in the body30. Our results show a significant decrease in PON-1 activity in the old smoke (OS) group when compared to young smoke group (YS) and middle-aged smoke (MS) group, indicating an increase in oxidative stress linked to cigarette smoke exposure. Further, it also suggests that the effect of cigarette smoke may be more pronounced in old-age rats as the antioxidant abilities decline with advancing age.
Advanced oxidation protein products (AOPP) can be found in biological fluids and can be an ideal method for identifying and assessing the extent of oxidant-mediated protein degradation because plasma proteins are the first target of free radicals. Therefore, they can be used as a marker of the degree of protein damage in oxidative stress31. The oxidative stress caused by cigarette smoke may be the reason for the increased AOPP content in the present study.
It has been shown that the protein carbonyl is a persistent oxidative modification of protein structure, most often, reactive oxygen species (ROS) cause protein modifications through carbonylation32. Indirect ROS-induced protein carbonylation can occur through lipid peroxides (for Cys, His, and Lys) or even glycation/glycoxidation (for Lys) caused by reactive oxygen species (ROS). Direct ROS attack of exposed side chains of amino acid residues such as Pro, Arg, Lys, and Thr causes carbonylation33. Since protein carbonylation is thought to be a stable and irreversible alteration and is a prominent byproduct of protein oxidation, it is frequently used as a marker to assess cellular oxidation. The increased protein carbonylation may be explained by the increase in oxidative stress, hence increasing the level of ROS in the cell as a result of cigarette smoke.
Sialic acid (SA), a terminal part of the non-reducing end of carbohydrate chains of glycoproteins and glycolipids, is one of the substances targeted by oxidative stress. SA is a necessary component of all cell membranes and is crucial for preserving the integrity, permeability, and structure of the membrane. Additionally, SA affects the transport mechanism, antigenicity, cellular adhesiveness, certain hormones’ actions, and the catalytic qualities of enzymes. Changes in sialic acid content have been linked to oxidative stress brought on by several reasons like inflammatory processes34. The increase in sialic acid level in our study may indicate an increase in the oxidative stress caused by cigarette smoke and the resultant increase in inflammation.
MDA is a marker of lipid peroxidation that is produced with the rise in oxidative stress in cells. The cause behind the significant rise in MDA observed in erythrocytes is the increase in free radicals after inhalation of cigarette smoke. Cigarette smoke contains ROS and reactive nitrogen species (RNS) producing various free radicals that increase lipid peroxidation reactions in cells35. The resultant increase in MDA in cells and overall reduction in antioxidant levels is highly toxic to health, especially for the lungs which gives rise to inflammation and lung diseases.
Limitations
This study provides important insights into the redox imbalance induced by passive cigarette smoke exposure in male Wistar rats; however, several limitations should be considered while interpreting the findings. First, the sample size was relatively small (n=36), which, while sufficient for preliminary analysis, may limit the statistical power to detect subtle biochemical changes or subgroup variations. Secondly, the exposure model involved controlled passive smoke exposure in a laboratory setting, which may not precisely mimic the chronic, variable exposure encountered in real-world human scenarios. Additionally, the duration of exposure was limited to 30 days, and the long-term effects of sustained redox imbalance could not be evaluated. Finally, while multiple oxidative stress markers were assessed, the study did not include functional or histopathological endpoints (e.g. tissue damage, behavioral outcomes), which could have provided a more comprehensive understanding of physiological consequences. Despite these limitations, the study establishes a foundational link between passive cigarette smoking and oxidative stress in vivo. Future studies with larger, more diverse animal cohorts, longer exposure durations, and broader endpoints are warranted to validate and expand upon these findings.
CONCLUSIONS
Our results provide a comparative analysis of the effect of passive smoking on different age groups of rats. We demonstrated that the oxidative stress caused by cigarette smoke was highest in the old-age rats, intermediate in the middle-aged rats, and least in the young rats. These data suggest that the ability to fight oxidative stress caused by passive smoking may be directly linked to the age of the rat, and seems to decline with the advancing age. It is highest in young rats and least in old rats. Further studies are needed in this regard to uncover possible reasons being responsible for such an age-dependent effect of passive smoking. Moreover, the present data from this study may be used in understanding similar effects of passive smoking in humans.


