INTRODUCTION
In modern medicine, risk assessment plays an important role in healthcare and clinical practice. Gender is one such risk factor known to modify the course of many diseases and illnesses. Development of risk assessment for treatment planning can be understood easily if gender differences can be established in the initiation, progression and outcome of many diseases1.
Historically, gender susceptibility was not a major consideration during diagnosis and treatment planning. However, it is now becoming an integral part of the diagnosis, therapy, prevention of diseases, in many health policies and clinical care. The World Health Organization had put forward a working group in 1996 to highlight gender issues in medicine and the importance of gender specific research. Several other international organizations that have promoted gender medicine include International Society of Gender Medicine (IGM), European Society of Gender Health and Medicine, The Comparative Gender Studies Committee of the International Comparative Literature Association (ICLA) and The Foundation for Gender-Specific Medicine thereby drawing global attention to gender disparities in health2.
Risk factors that affect gender susceptibility may include social, behavioral, biological, and genetic factors. Examples of gender susceptibility in disease prevalence are documented in the literature and explained by these factors. Social factors are often reflected in tropical diseases such as malaria, where men are more likely to be exposed to mosquitoes in certain industries such as forestry or mining3. Behavioral factors like tobacco smoking and alcohol drinking have been implicated as risk factors for diseases like gastroesophageal reflux disease in men4. Biological factors can include the protective role of female hormones like endogenous estrogen in cases of diseases like myocardial infarction, where men are overall 4.6 times more likely to have the disease with respect to premenopausal women5. Women are 2.7 times more likely to acquire an autoimmune disease compared to men due to the protective role of androgen, and also the hormone estrogen is a potential autoimmunity stimulator6. When considering genetic factors, males have a higher risk for lone atrial fibrillation (LAF) due to dominant Mendelian inheritance. X-linked recessive inheritance could be a factor for increased incidence of sporadic lone atrial fibrillation in men7.
For oral diseases that are multifactorial in origin, risk factors play a major role in diseases prevention and treatment. For example, controlling a small number of risk factors like diet, plaque and smoking, can go a long way in prevention and control of diseases like dental caries and periodontal diseases8. Many oral diseases like oral cancer, oral lichen planus, leukoplakia were found to be high among the Indian population as many deleterious habits like smoking, drinking and chewing tobacco products, are strongly associated with the development of these oral lesions9.
The prevalence of oral diseases was found to be high in India10. In the case of dental caries, the prevalence was found to be 84.7% in those aged 65–74 years and root caries were 3.9% and 5.4% predominant in the age groups 35–44 and 65–74 years, respectively. For periodontal disease, loss of attachment of 4–5 mm depth affected 42.2% of the population in those aged 35–44 years and 60.6% in the ages 65–74 years. Percentage of malocclusion was found to be 1.6%, 23.6% and 23.9% in children aged 5, 12 and 15 years, respectively. Also, 42% of adults aged 35–44 years in the country had malocclusion. Adults aged 65–74 years were found to have the highest prevalence of oral mucosal lesions in India, with oral cancer in 0.4% and leukoplakia in 3.1% of the population. Lichen planus was reported in 0.4% of adults aged 35–44 years and 0.5% aged 65–74 years. In India, 80000 new cases of oral cancers are detected every year, out of which 95% are squamous cell carcinoma. Complete or partial edentulism or absence of teeth was found in the age group 65–74 years (30% was found to be completely edentulous). However, only 0.8% of the individuals aged 35–44 years were found to be edentulous. This means that teeth are lost mainly in old age10.
During the last 30 years, several studies have been conducted worldwide to investigate the possible gender variations in oral health status and behavior among populations of various age groups and characteristics. It has been found that there exists a disparity in oral disease prevalence when genders are compared, and many countries have reported this difference with underlying factors influencing this11,12.
More importance has to be given to gender and oral health in India, for the delivery of efficient patient care, management of health services, public health awareness, and health policy making13. The compilation of data on gender susceptibility in India is much warranted to appreciate the gender differences of oral health in the Indian population. Therefore, a metadata exploration was performed to ascertain gender susceptibility to the five major oral diseases including dental caries, malocclusion, oral cancer, tooth loss, gingivitis and periodontitis, which are reported to have a high prevalence in India.
METHODS
This metadata exploration was performed based on information retrieved from systematic reviews and meta-analyses of point prevalence studies and national oral health surveys with gender specific data. Literature search was performed on Pubmed/Medline and Scopus databases with database-specific search strategies. Search strategy was developed to identify systematic reviews and/or meta-analyses on five common oral diseases in India: dental caries, periodontal disease, gingivitis, oral cancer, tooth loss, and malocclusion. Filters (Systematic Reviews; Meta-analyses) were applied to restrict the results to potentially eligible studies. Only studies published in English were included. Articles of any other study design (e.g. cross-sectional, case-control, cohort, experimental designs, narrative reviews, case reports etc.) were excluded.
The retrieved articles from the two databases were imported to the search management software Covidence. After removal of duplicates, two authors (RV and VK) initially screened the titles and abstracts, based on eligibility criteria and then a full-text screening was performed. Articles not fitting the inclusion criteria were excluded with reasons. Data extraction from the included studies was then undertaken by a third person (KK) using a customized template. The following information was extracted from each article: Authors, country, state, study design, article inclusion dates, disease (outcomes) under study, age group, type of population, inclusion/exclusion criteria, sample size, sampling strategy and tool (index) used for outcome measurement.
Metadata analysis
Since different levels of evidence were used (i.e. systematic reviews, scoping reviews, meta-analyses, pooled prevalence etc.), a qualitative synthesis of data (summary) was undertaken. Data synthesis was done by extracting data related to description of gender-related results in the article and effect size (if any), which included pooled prevalence, odds ratio, relative risk, or aggregate percentages.
RESULTS
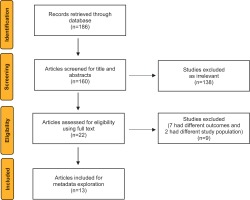
A total of 186 studies were retrieved through database search. After removal of duplicates (n=26), 160 studies were screened based on title and abstract, of which 138 studies were deemed irrelevant to the review requirements. Among the 22 potentially relevant studies screened using full-text, 9 studies were excluded (7 studies measured an outcome other than the diseases of interest and 2 studies had a different study population). Finally, a total of 13 studies were included for metadata exploration (qualitative synthesis) (Figure 1).
Table 1 shows the characteristics of included studies and Table 2 outlines the outcomes and summary measures for each included study.
Table 1
Characteristics of included studies
| Authors and Year | Country of affiliation of corresponding author | Study design | Period of articles included | Disease under study | Age range | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|
| Kirthiga et al.14 2019 | India | Systematic review and meta-analysis | 1 July 2016 – January 2019 | Dental caries (early childhood caries) | From birth until 6 years of age | All preschool children, regardless of gender, race, health status, geographical location, or socioeconomic status (SES), from birth until six years of age (<72 months old) were included. | Children with special healthcare needs were excluded. |
| Janakiram et al.13 2018 | India | Any other review | January 2000 – April 2016 | Dental caries | 5, 12, 15, 35–44 and 60–74 years | Point/period prevalence studies on dental caries prevalence or caries experience using deft/DMFT index done in India from January 2000 – April 2016; Studies within the index ages (5, 12, 15, 35–44 and 60–74 years). Only studies published in English were included. | The search was restricted to articles published in PubMed database and did not include other sources, government reports, or grey literature. |
| Lukacs17 2011 | United States | Any other review | Till 2011 | Dental caries | 5–7 years, 12–15 years, adults (mature and older adults) | A literature survey yielded extensive data on caries rates in India, Nepal, Bangladesh, and Sri Lanka (South Asia). Most studies were restricted to one or two age groups, typically one between 5 and 7 years and another between 12 and 15 years. Fewer studies examined caries prevalence in adults, though some included mature and older adults in the study group. | |
| Pentapati et al.15 2019 | India | Systematic review and meta-analysis | Until 1 January 2018 | Dental caries (root caries) | Adults aged >18 years were included | Studies designed as a cohort or cross-sectional observational studies, studies where prevalence data can be extracted or calculated, and studies conducted in adults aged >18 years were included. Only articles published in English were included. | Conference proceedings, editorials, and letters were excluded. |
| Srivastava et al.16 2012 | India | Systematic review | January 1990 – December 2009 | Dental caries (dental caries in geriatric population) | Elderly population in India, i.e. aged ≥60 years | The inclusion criteria for this review were: studies conducted in elderly population in India, i.e. aged ≥60 years, published in English. The period was January 1990 – December 2009 since we assume that data over the last two decades would provide the trend of prevalence of dental caries over the years. | Electronic search for dental caries in the elderly yielded 26 references, of which 6 were retained. The rest of the studies were excluded because they were hospital based, or were carried out in children or adults aged <60 years. |
| Bhanu Prasad et al.19 2017 | India | Any other review | Until 2016 | Oral cancer (squamous cell carcinoma) | Aged ≤20 years | Published articles describing head and neck squamous cell carcinoma and treatment aged ≤20 years, fully published case reports or case series. | Articles on pathology and genetics alone without report on treatment and clinical outcomes were excluded from the analysis. |
| Shrestha et al.20 2020 | Denmark | Any other review | Before 3 March 2016 | Oral cancer | Aged ≥15 years | The inclusion criteria were original studies, studies reporting prevalence or incidence rates, population-based studies, published in English, and studies involving humans | Exclusion criteria were studies conducted outside LMICs, studies with no information about oral cancer, review studies, reports and duplicates. |
| Sinha et al.21 2016 | India | Systematic review and meta-analysis | Until 30 June 2015 | Oral cancer (smokeless tobacco-associated cancers) | Adult Indians aged ≥35 years | A list of cancers that were most likely to be associated with SLT use was drawn and these were selected for further study. | Duplicate data, no control, small sample, no estimate, not site specific, no adjusted estimate. |
| Rao et al.22 2013 | Australia | Any other review | 2000–2012 | Oral cancer | All ages | Literature search for this review was conducted on Medline for articles on oral cancer from Asian countries. The published articles available were mainly from India, Pakistan, Sri Lanka and Taiwan. | Articles were excluded if the data on OC were combined and presented as head and neck cancer. |
| Janakiram et al.18 2020 | India | Systematic review and meta-analysis | 1987–2018 | Periodontal disease (gingivitis, periodontitis, mild to moderate periodontitis, severe periodontitis) | Adults aged >18 years | Included studies assessed the prevalence and severity of periodontal disease among participants aged >18 years using the Community Periodontal Index of Treatment Needs (CPITN) or Community Periodontal Index (CPI). | Studies utilizing convenience sampling were excluded from quantitative analysis. |
| Mehta et al.23 2020 | India | Systematic review and meta-analysis | 1 January 2000 – 31 December 2019 | Malocclusion | Indian children and adolescents aged <20 years | This systematic review concentrated on short listing the population or school based cross-sectional studies conducted on Indian children and adolescents (aged <20 years) assessing prevalence of malocclusion according to different indices and classifications. | Studies that did not report the prevalence of dental malocclusion, sample size, abstracts submitted to conferences, case report studies, seminars, case control studies and clinical trials not providing an accurate estimation of the prevalence, studies that did not obtain a minimum score of quality assessment and studies with a participant population aged ≥20 years were also excluded. |
| Balachandran et al.24 2021 | India | Systematic review and meta-analysis | 2000–2020 | Malocclusion | Aged 8–15 years | This systematic review was limited to cross sectional studies published in English since 2000. In the included studies, the prevalence of malocclusion was assessed using Dental Aesthetic Index (DAI) or Angle’s classification of malocclusion (including Dewey’s modification) | Studies that assessed the prevalence of malocclusion in children with special healthcare needs (e.g. visually challenged) or those with medically compromised subjects (e.g. children with hemophilia), studies that assessed the orthodontic treatment needs rather than prevalence of malocclusion were not included. |
| Venkat et al.25 2021 | India | Systematic review and meta-analysis | Studies published from 1 January 2010 – August 2019 | Tooth loss | Adults aged ≥18 years | The studies which assessed the prevalence and oral health status of tooth mortality among adults aged ≥18 years to assess the prevalence of tooth mortality. | Studies that did not meet the inclusion criteria were excluded. |
Table 2
Summary statistics of included studies
| Authors and Year | Sampling (random/non-random/no information available) | Sample and Number | Outcome measurement Index (DMFT, CPI other indices etc.) | Description of outcome in the manuscript | Effect measures (odds ratio, relative risk, p value, pooled prevalence) |
|---|---|---|---|---|---|
| Kirthiga et al.14 2019 | No information available | Upper middle-income countries 937 High-income countries 2727 | DMFT, International Caries Detection and Assessment System (ICDAS), five egrade caries diagnostic system | Of the 19 sociodemographic factors, gender (male) and low household income were found to be frequently implicated, in most studies. Among the studies grouped under UMI countries, the factors found to have a positive association with ECC (OR greater than one) included the gender male. | Pooled odds ratio (Male): Upper middle-income countries 1.26% (95% CI: 0.85–1.88); High-income countries 0.98% (95% CI: 0.80–1.19). |
| Janakiram et al.13 2018 | No information available | deft/DMFT index | Males had slightly higher prevalence at 5 and 12 years and females had a higher prevalence in the older age groups. | Prevalence: 5 years: males 52%, females 49% 12 years: males 51%, females 50% 15 years: males 59%, females 61% 35–44 years: males 76%, females 80% 65–74 years: males 84%, females 85% | |
| Lukacs17 2011 | No information available | Males 25265 Females 521696 | DMFT | A male bias in caries prevalence was least common (9.5% of comparisons; 6 of 63) and females expressed significantly greater caries prevalence than men in 16 of 63 comparisons (25%). | Caries prevalence among Nepali school children aged 5–6 years: females 63%, males 70% aged 12–13 years: females 43%, males 38% |
| Pentapati et al.15 2019 | No information available | Most studies summarized the results in terms of percentage decayed or decayed filled teeth and/or root caries index. | Concerning gender, minimal difference in the prevalence estimates was seen between males (34.5%) and females (33.3%). | The pooled prevalence among males was 34.5% (95% CI: 28.2–40.9) and among females was 33.3% (95% CI: 26.3–40.3) with no statistical difference between them. | |
| Srivastava et al.16 2012 | No information available | DMFT Index | The present review shows that the burden of dental caries is high in the elderly population in India. | Weighted prevalence of dental caries experience was 83.6% in 2000–2004 and 82.3% in the period 2005–2009. | |
| Bhanu Prasad et al.19 2017 | No information available | 217 Males 66 Females 38 | In the present study 66 (63.46%) patients were male and 38 (36.54%) were female, with a male preponderance. Sex ratio was skewed in favor of males with ratio of 1.7:1. | Overall survival Males: 74.64% (95% CI: 0.58–0.85) Females: 67.71% (95% CI: 0.45–0.82) Disease free survival Males: 64.06% (95% CI: 0.46–0.77) Females: 47.24% (95% CI: 0.27–0.64) | |
| Shrestha et al.20 2020 | No information available | Sample sizes ranged from 486 to 101761 with a total of 213572 study participants | This review reveals oral cancer prevalence and incidence from 0.12 to 4.12 per 1000, and incidence was reported to be 8.5 per 100000 people per year. | The risk ratio for women chewing tobacco 10 times or more a day was 9.2 (95% CI: 4.5–18.7) | |
| Sinha et al.21 2016 | No information available | The annual number of SLT attributable cancer cases was 49192 for mouth (60% of all oral cancers) | In the case of oral cancer, the pooled OR for women was much higher compared to men (12.0 vs 5.2). | Pooled OR (95% CI): Males: 5.16 (4.49–5.94) Females: 12.03 (9.49–15.25) | |
| Rao et al.22 2013 | No information available | Considering all the age groups, men are more affected than women. | Tobacco being an independent risk factor, the relative risk of occurrence of OC in tobacco users is 11 times that of people who never used tobacco. Odds ratio (OR) of 2.5–2.8 has been calculated for development of OC among farmers in India. | ||
| Janakiram et al.18 2020 | Total: 68140 Males 31030 Females 28045 | Community Periodontal Index of Treatment Needs (CPITN) or Community Periodontal Index (CPI) | When severity of periodontitis was stratified by age, sex and type of population, males had higher proportion of severe periodontitis (19.3%; 95% CI: 11.3–28.8; 9 studies) than females (14.4%; 95% CI: 7.1–23.6). | Pooled prevalence Gingivitis Males: 75.0% (95% CI: 74.6–75.5) Females: 77.3% (95% CI: 76.8–77.7) Periodontitis Males: 42% (95% CI: 20.3–65.8) Females: 34.4% (95% CI: 2.4–60.2) Mild to moderate periodontitis Males: 17.8% (95% CI: 5.8–33.8) Females: 16.7% (95% CI: 4.2–34.4) Severe periodontitis Males: 19.3% (95% CI: 11.3–28.8) Females: 14.4% (95% CI: 7.1–23.6) | |
| Mehta et al.23 2020 | No information available | 71409 children | Dental Aesthetic Index (DAI), Index of orthodontic treatment needs, Angle’s classification, Terminal plane relationship of second primary molars | Statistically significant higher proportion of malocclusion was seen among boys (43.6%; 95% CI: 35.5–51.9) compared with girls (28.1%; 95% CI: 23.1–33.3, p<0.001). We observed a higher prevalence of malocclusion among Indian boys compared to girls. | Pooled prevalence 8.4% (95% CI: 25.02–31.9) from a sample of 71409 children. Statistically significant higher proportion of malocclusion was seen among boys (43.6%; 95% CI: 35.5–51.9) compared with girls (28.1%; 95% CI: 23.1–33.3, p<0.001) |
| Balachandran et al.24 2021 | No information available | Total 97959 Males 40456 Females 36938 | Dental Aesthetic Index (DAI) or Angle’s classification of malocclusion (including Dewey’s modification comprising further subdivisions of Angle’s class I and III) | Males had a higher proportion of malocclusion (36.20%; 95% CI: 36.12–36.28; 33 studies, 40456 participants) than females (31.98%; 95% CI: 31.93–32.03; 33 studies, 36938 participants). When the mean DAI score was stratified by gender, the prevalence of malocclusion among males and females were 21.46 (95% CI: 21.36–21.57; 15 studies, 9547 participants) and 21.52 (95% CI: 21.41–21.64; 15 studies, 8276 participants), respectively. | Pooled prevalence based on proportion Males 36.20% (95% CI: 36.12–36.28) Female 31.98% (95% CI: 31.93–32.03) Pooled Prevalence based on DAI score Males 21.46% (95% CI: 1.36–21.57) Females 21.52% (95% CI: 1.41–21.64) |
| Venkat et al.25 2021 | No information available | 27324 adults | DMF, DSTN | Overall prevalence for tooth loss was found to be higher in females than in males. Females had higher partial tooth mortality (48.2%), whereas males had higher complete tooth mortality (20.2%). | Pooled prevalence of partial edentulism Males 41.1% (95% CI: 39.8–42.4) Females 48.2% (95% CI: 46.4–50.0) Complete edentulism Males 20.2% (95% CI: 19.1–21.2) Females 18.6% (95% CI: 17.2–20.2). |
Dental caries
A total of five studies explored the metadata of dental caries prevalence13-17, two of which focused exclusively on early childhood caries14 and geriatric population, respectively16, while another three studies reported caries prevalence according to different age groups. Caries burden based on WHO age groups was reported in two studies13,17 while one study reported the prevalence of root caries among adult population. All studies used DMFT as the main indicator to assess caries status, while one study additionally reported prevalence based on ICDAS system14.
Majority of studies reported an increased prevalence of dental caries among males. The effect measures used varied across studies. While males had 1.26 times odds of having ECC compared to females in a study done by Kirthiga et al.14, similar observations (52% compared to 48%) were noted in a metadata analysis of those aged 5 years by Janakiram et al.13 and Lukacs17. Root caries showed no statistically significant difference between males (34.5%) and females in the adult population (33.3%)15. The study by Lukacs17 in South Asia showed that among the adult population, prevalence of caries among males was lower compared to females17.
Gingival and periodontal disease
A study by Janakiram et al.18 explored the metadata of periodontal disease prevalence. This study reviewed literature from 1987–2018, which assessed the prevalence and severity of periodontal disease in individuals aged >18 years using the Community Periodontal Index of Treatment Needs (CPITN) and Community Periodontal Index (CPI). The analysis included gender specific data on gingivitis, mild to moderate periodontitis, and severe periodontitis. In this study, the pooled prevalence of periodontal disease was found to be higher in males (42.2%; 95% CI: 20.3–65.8) than in females (34.4%; 95% CI: 12.4–60.2). Gingivitis was reported to be more among females with pooled prevalence of (77.3%; 95% CI: 76.8–77.7) compared to males (75.0%; 95% CI: 74.6–75.5). Subgroup analysis revealed higher proportion of severe periodontitis among males (19.3%; 95% CI: 11.3–28.8) than females (14.4%; 95% CI: 7.1–23.6). While moderate to mild periodontitis was noted to have a slight increase in prevalence among the male population (17.8%; 95% CI: 5.8–33.8) in comparison to females (16.7%; 95% CI: 4.2–34.4)18.
Oral cancer
Four studies explored the metadata of oral cancer prevalence in India19–22, of which one explored smokeless tobacco-associated oral cancers in adult Indians aged ≥35 years while another19 was based on pediatric head and neck squamous cell carcinoma in people aged ≤20 years. Two other studies focused entirely on prevalence and incidence of oral cancer in middle- and low-income countries in people aged ≥15 years20 and among people of all age groups22, respectively.
The majority of studies reported an increased prevalence of oral cancer among males. Sex ratio was skewed in favor of males with ratio of 1.7:1 in the study by Bhanuprasad et al.19 in patients aged ≤20 years with head and neck squamous cell carcinoma. Considering all the age groups, men were found to be more affected than women. In another study by Sinha et al.21, on smokeless tobacco-associated cancers, the pooled odds ratio was found to be higher in women than in men (12.0 vs 5.2).
Malocclusion
Two studies explored the metadata on the prevalence of malocclusion23,24. A study by Mehta et al.23 focused on Indian children and adolescents aged ≤19 years and another by Balachandran et al.24 focused on children aged <15 years. Both studies23,24 used Index of orthodontic treatment needs (IOTN) and Angles classification as the main indicators to assess malocclusion, and one study23 additionally assessed Terminal plane and Dental Aesthetic Index (DAI). Statistically noteworthy (p<0.001) proportion of malocclusion was found in boys (43.6%; 95% CI: 35.5–51.9) than in girls (28.1%; 95% CI: 23.1–33.3) in both studies.
Tooth loss
One systematic review and meta-analysis was done to assess partial and complete edentulism in India25. Overall prevalence for tooth loss was found to be higher in females than in males. Pooled prevalence of partial edentulism was 41.1% (95% CI: 39.8–42.4) for males and 48.2% (95% CI: 46.4–50.0%) for females while that of complete edentulism was 20.2% (95% CI: 19.1–21.2) for males and 18.6% (95% CI: 17.2–20.2) for females25.
DISCUSSION
Data on risk factors associated with oral diseases are available in the literature, in which gender susceptibility is one of the most discussed. As behavioral, sociocultural and biological factors are associated with gender, region-wise data need to be explored to design risk-based interventions. This metadata exploration identified 13 studies from India to investigate the gender susceptibility of oral diseases in the country.
The majority of studies13-16 on dental caries in the Indian population reported an increased prevalence of dental caries among males. This is contrary to the existing paradigm where caries rates are higher in women than in men. The causes cited include factors such as low maternal education and socioeconomic status, and unemployment of the mother14. Only one study from India showed a female susceptibility, with reported cause being female sex hormones like estrogen and estradiol complementing the onset of puberty in girls17. When further exploring the literature, the reasons cited include various biological factors like the prior eruption of teeth in girls, and the extended exposure to the cariogenic oral environment, different salivary composition, salivary flow rate variation, hormonal fluctuation, pregnancy, differences in the oral microflora, and variation in the quality of tooth enamel. Genetic factors can be considered in controlling enamel formation leading to higher prevalence in females and there can also be a genetic variation where a deficiency of an enamel forming protein Amelogenin, leads to disruption in the formation of enamel matrix and hence increased risk for caries26. Behavioral factors include dietary habits, accessibility of women to food supplies and frequent in between snacking, especially during food preparation27. At the time of the agricultural revolution, the division of labor resulted in women being exposed to gathering food items and men to consuming meats, thereby men were exposed to considerably lower sugar content28. Social factors can include male child getting more healthcare attention and nutritious food, the custom of fasting among Hindu women, and the false belief that limitation of one’s diet during the time of pregnancy can result in a less problematic birth can definitely lead to dietary imbalances, which can eventually result in caries formation29. The reason for higher prevalence of dental caries in males needs to be explored in the Indian population.
When considering periodontal disease, the Indian data agreed with the world data, where the severity of periodontitis was found to be higher in males than females18. The reasons can be attributed to biological factors like immunosuppressive role of testosterone and progesterone, as well as the immune-enhancing impact of estrogens in women30. Metabolic diseases like diabetes can also be a predisposing factor for periodontitis in men31. Environmental factors can have a huge impact on periodontal flora which include factors like diet and nutrition, education, and dental care32. Behavioral factors like tobacco chewing and smoking (OR=4), poor maintenance of oral hygiene, and socioeconomic status are risk factors predisposing the males to higher odds of developing severe periodontal disease than females30. There are gingival inflammatory conditions found in females which are related to hormonal conditions, such as pregnancy gingivitis that can explain higher prevalence of gingivitis in women.
Indian studies reported females to have higher prevalence of tooth loss compared to males25. This is in agreement with the existing paradigm. A few studies have observed that there is a significant relationship between gender and various classes of partial edentulism. This can be understood based on greater susceptibility to caries among women. Important risk factors for edentulism include biological and social factors33. Various socioeconomic factors, psychological factors, use of more sugars, behavioral factors like maintenance of oral hygiene behavior and damaging behaviors such as smoking and diet, can lead to caries and periodontitis and eventually tooth loss34. Smoking is an independent risk factor for periodontitis and tooth loss, which is reported to be a major factor in edentulism of the male population. Socioeconomic factors also influence gender susceptibility, like education level, occupation, and income, which can be correlated to the use of dental care and dental insurance. Biological factors like changing hormonal levels can be related to women’s reproductive functions like menstruation, pregnancy, and parity, which could disturb the periodontium adversely. The condition of the periodontium, in turn, influences whether teeth should either be retained or extracted. Tooth loss can also be related to multiple pregnancies due to increased hormonal fluctuations. Also, menopause and accompanying osteoporosis can lead to periodontitis and tooth loss in women34.
The Indian data reported that malocclusion was found to be higher in boys than in girls which is in line with the existing paradigm. Much of the noticeable malocclusions could also be connected with bullying and a lower selfe-steem among adolescents23. The increased susceptibility of malocclusion in those aged 8–15 years can be due to delayed growth spurts. Yet, females were found to be more likely to undergo orthodontic treatment than males due to their higher aesthetic requirements24.
When considering classification of malocclusion, it is noted that males have a tendency toward prognathism, whereas females demonstrated a tendency toward orthognathism and retrognathism. The most common and most frequent environmental etiologic factors of malocclusions are behavioral factors like parafunctions and dysfunctions (digit sucking, nail biting, breathing, and swallowing) rather than biological factors. Indian studies of malocclusion were assessed using dental aesthetic index indicating severity of malocclusion based on crowding and positions of teeth. Molar relations and skeletal malocclusion are given less weight in DAI35. In a study by Pruthi et al.36, in a North Indian adolescent population, the need for orthodontic treatment was found to be higher among boys, and a higher proportion of girls than boys were rated as having normal or minor malocclusion. In another study by Silvola et al.37, in a Finnish adult population, deep bite was associated with higher oral health impact profile in men. Females compared to males with Angles class III malocclusion had a smaller linear dimension in the maxilla, mandible, and also anterior facial heights, especially in the age of adolescence38. Boys also presented with a high number of Angles class II and class III type of malocclusion compared to girls39. However, a study done in Japan revealed that girls had a higher tendency for developing anterior crossbite and upper and lower crowding due to smaller sizes of their maxilla and mandible than in boys40.
For oral cancer the existing paradigm is that it is reported more in males. Similar trend is observed in Indian studies19,20,22 where a male predominance for oral cancer was noted compared to females. The reasons cited are based on behavioral factors such as, the usage of tobacco by 57% of men and 11% of women aged 15–49 years41. The National Family Health Survey 2015–2016 had reported that cancer cases among females are on the rise, however, the problem is still extensive amidst males42. Also, tobacco chewers were found to be more than smokers in the Indian subcontinent. Cancers involving the buccal mucosa and lower alveolus were often associated with chewing tobacco, whereas smoking and alcohol consumption has been linked with the higher occurrence of cancers involving the tongue43.
Biological factors like the deficiency of estrogen and the hormonal differences between the two genders justifies the reduced rate of oral cancer in women. Estrogen deficiency and elevated fasting glucose are risk factors for oral cancer43. However, in patients aged >50 years who are non-smokers, oral cancer was found to be greater in women than in men. Elevated rates of oral cancer were found in those who did not consume many fruits and vegetables and also in people with malnutrition44. Other risk factors include poor oral hygiene, and chronic irritation. The female patients were less exposed to factors like cigarettes, alcohol, and betel-nut chewing45.
A study by Krishna Rao et al.22 on epidemiology of oral cancer in Asia revealed that when all age groups were compared, men were at a higher risk than women, however, reversed male to female ratios of 1:2.0 and 1:1.56 were found in India (Bangalore) and Thailand. This could be explained by behavioral factors like lower prevalence of smoking and drinking habits among women or methodological factors in women like small sample size or lesser event rates. Increased incidence of oral cancer among women can be due to higher smoking associated risk in women (OR=3.2) compared to men (OR=1.8). Also, viral infections, such as HPV and oral hygiene, are other imperative risk factors. Factors like chewing tobacco along with consumption of alcohol and smoking, low socioeconomic status, and malnutrition, can also be a possible risk factor. These days items like gutkha and pan masala are easily accessible, and are used by children, men, and women alike. This in turn increases the risk for oral cancer22. In another study by Sinha et al.21 2016 on smokeless tobacco-associated cancers21, the pooled odds ratio was found to be greater in women than in men (12.0 vs 5.2).
To the best of our knowledge, this in one of the first studies of its kind to report a combined metadata on gender susceptibility for oral diseases in India and how it compares with that of global understanding.
Strengths and limitations
The strength of the study is the concept of ‘metadata exploration’ where we relied on data obtained from systematic reviews and/or meta-analyses of prevalence studies. However, a quantitative analysis of metadata was not possible due to the few studies included and the heterogeneity in assessment methods, hence descriptions and comparisons were qualitative.
CONCLUSIONS
The objective of the review was to explore the metadata regarding gender variations in oral diseases. Metadata from Indian studies on gender variations in dental caries contrasted with that of world data with majority of studies reporting an increased prevalence of dental caries among males than in females. The prevalence of periodontitis, however, was in agreement with the global data, where it was found to be higher in males compared to females. Also, most of the Indian studies showed a male predominance in oral cancer compared to females. Similarly, the Indian data showed that malocclusion was more prevalent among boys than in girls.